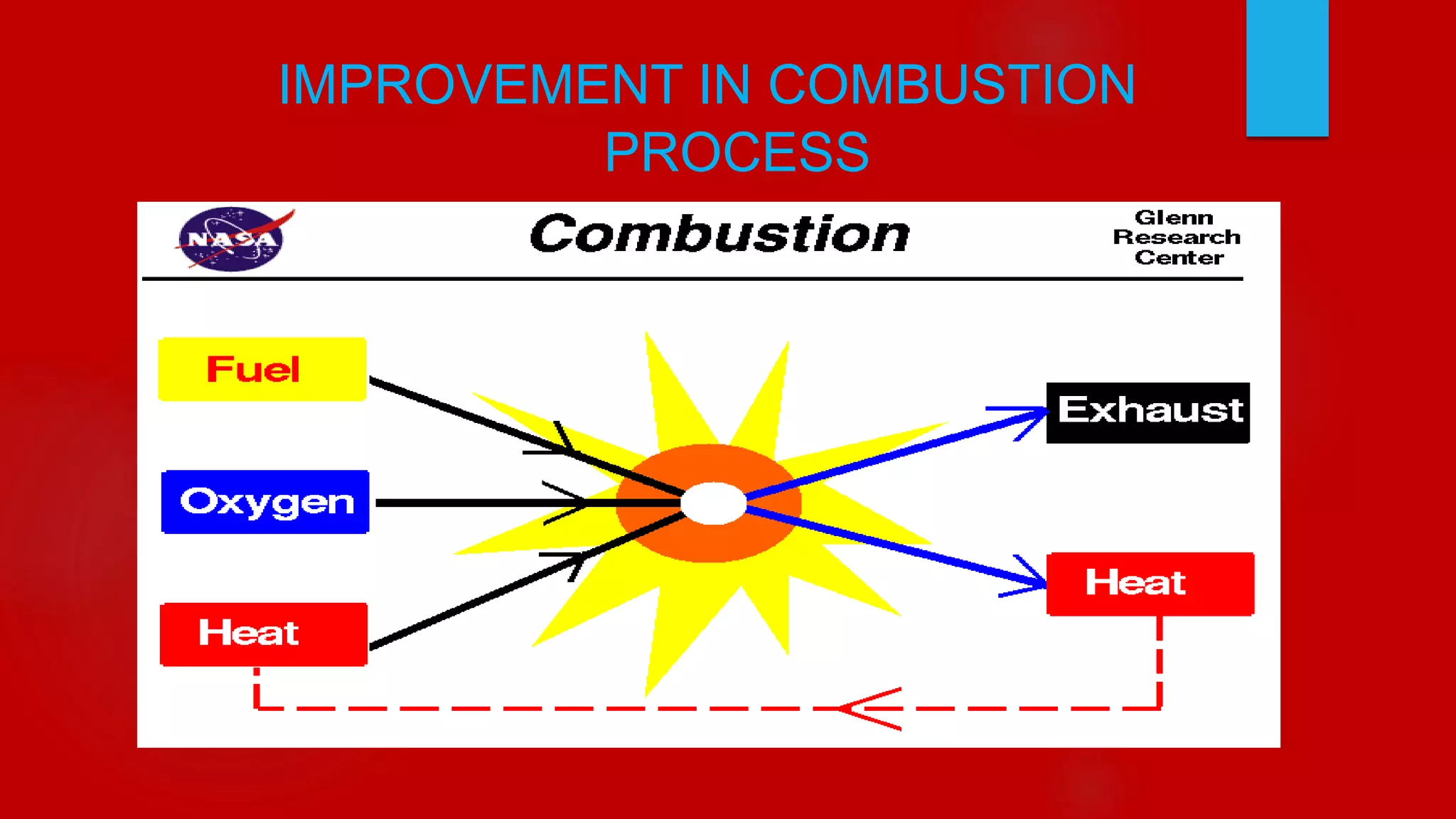
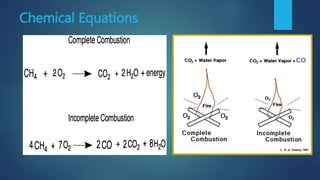
The document discusses improvement in combustion processes. It provides an overview of combustion and its effects on the environment. Emissions from combustion like CO2, CO, SO2, and NOx are outlined along with their effects such as global warming, smog, and acid rain. Methods to improve combustion efficiency and reduce emissions include optimizing combustion device design, using catalytic converters, and exhaust gas recirculation.