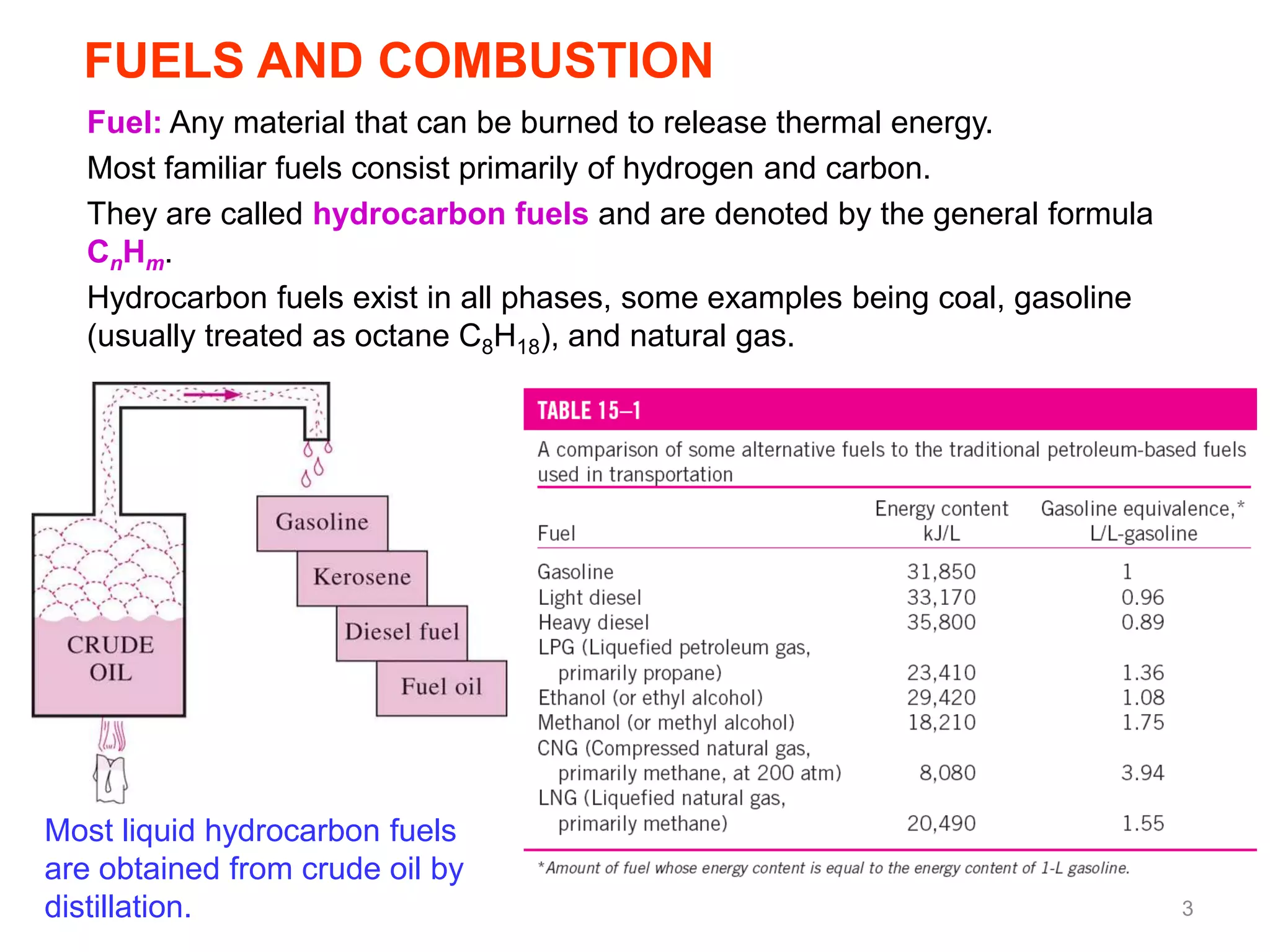
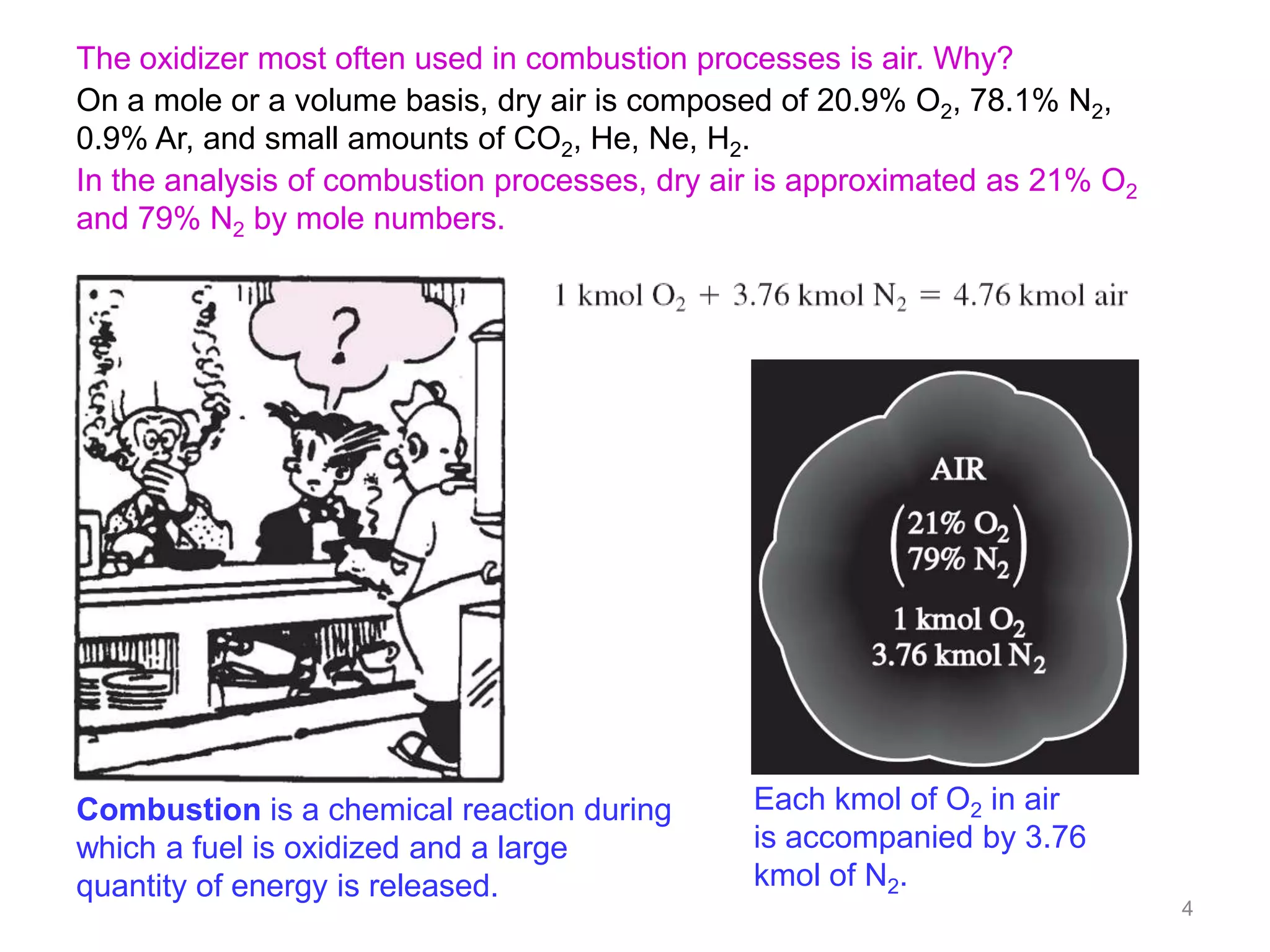
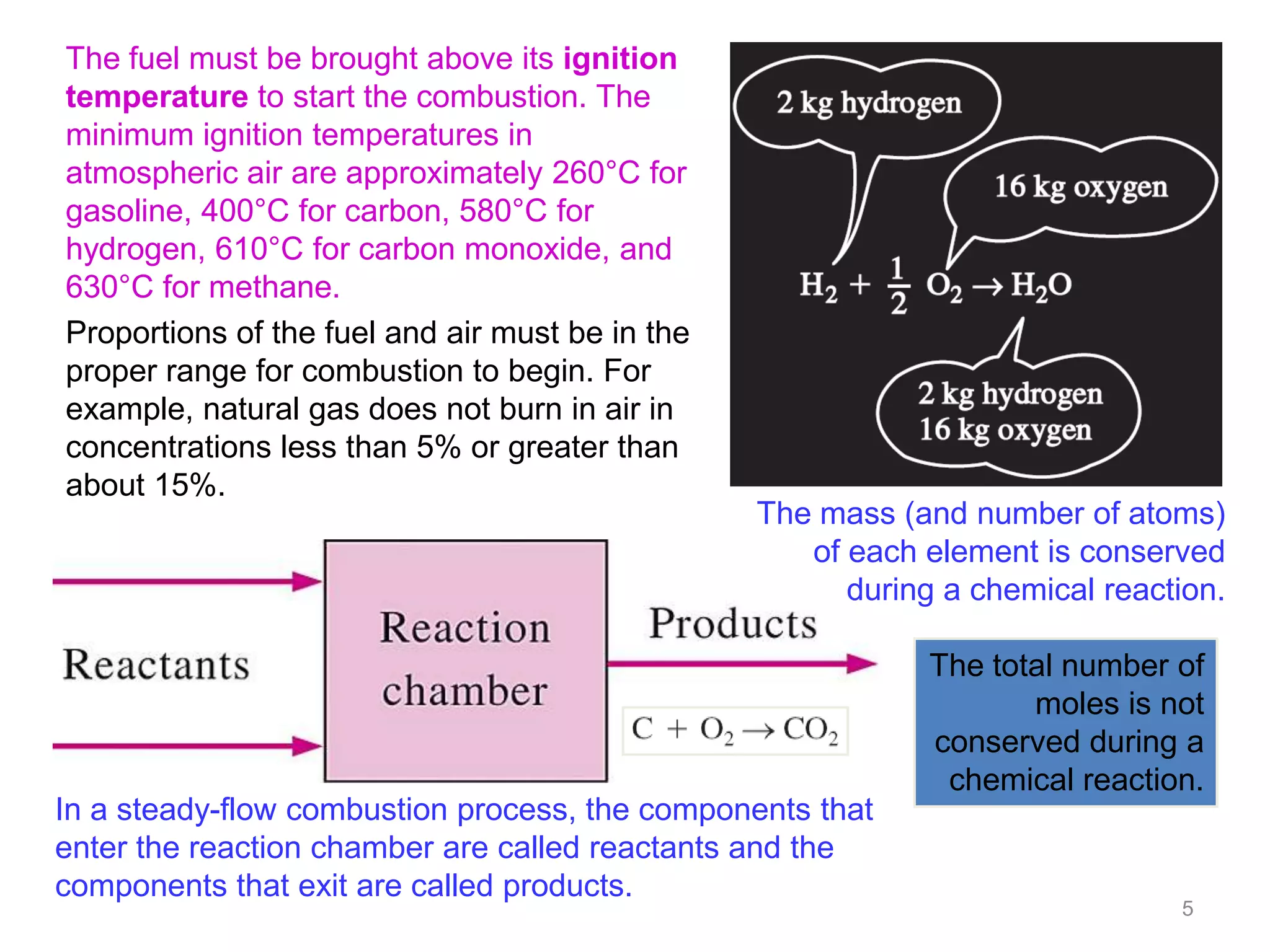
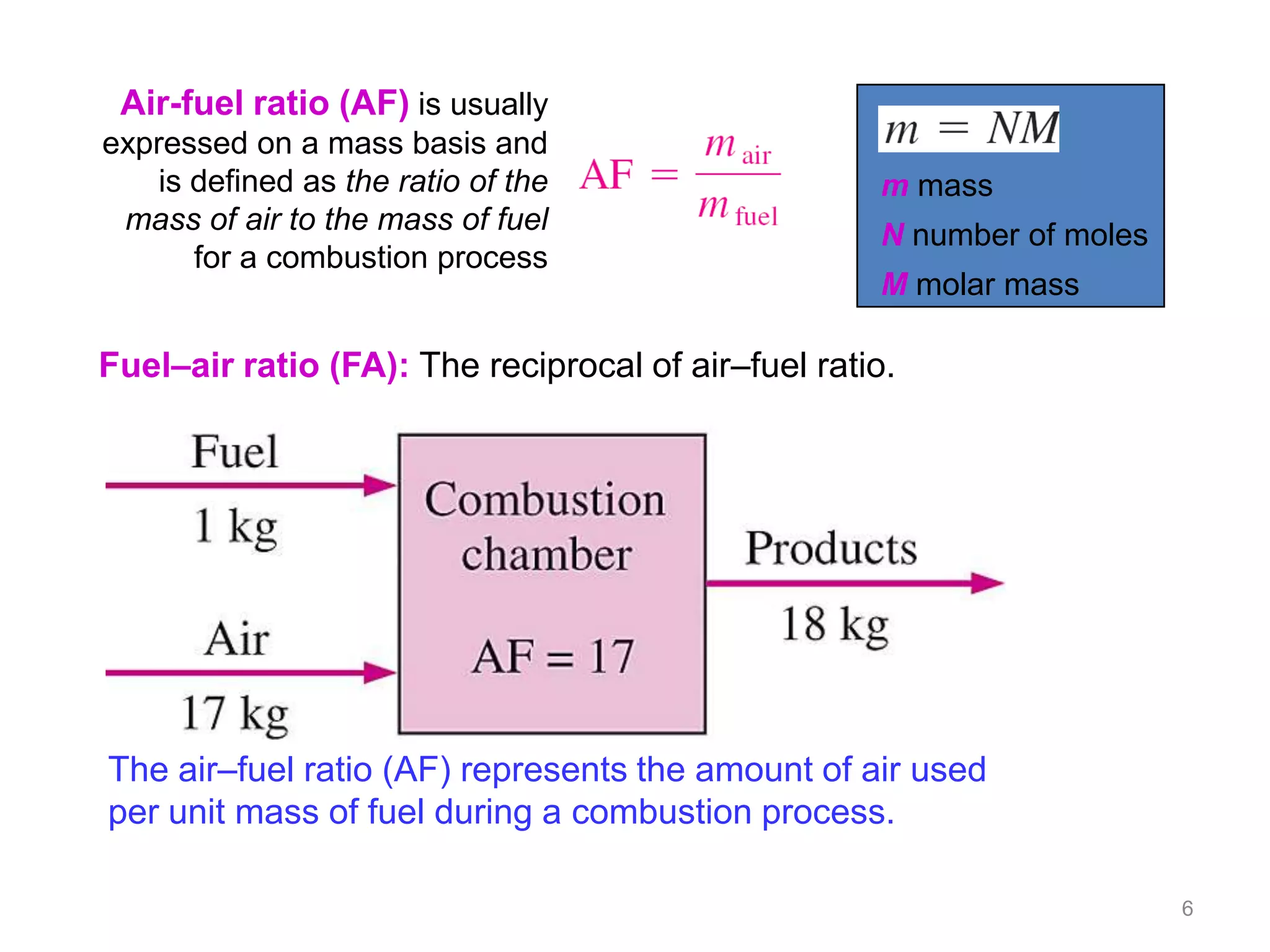
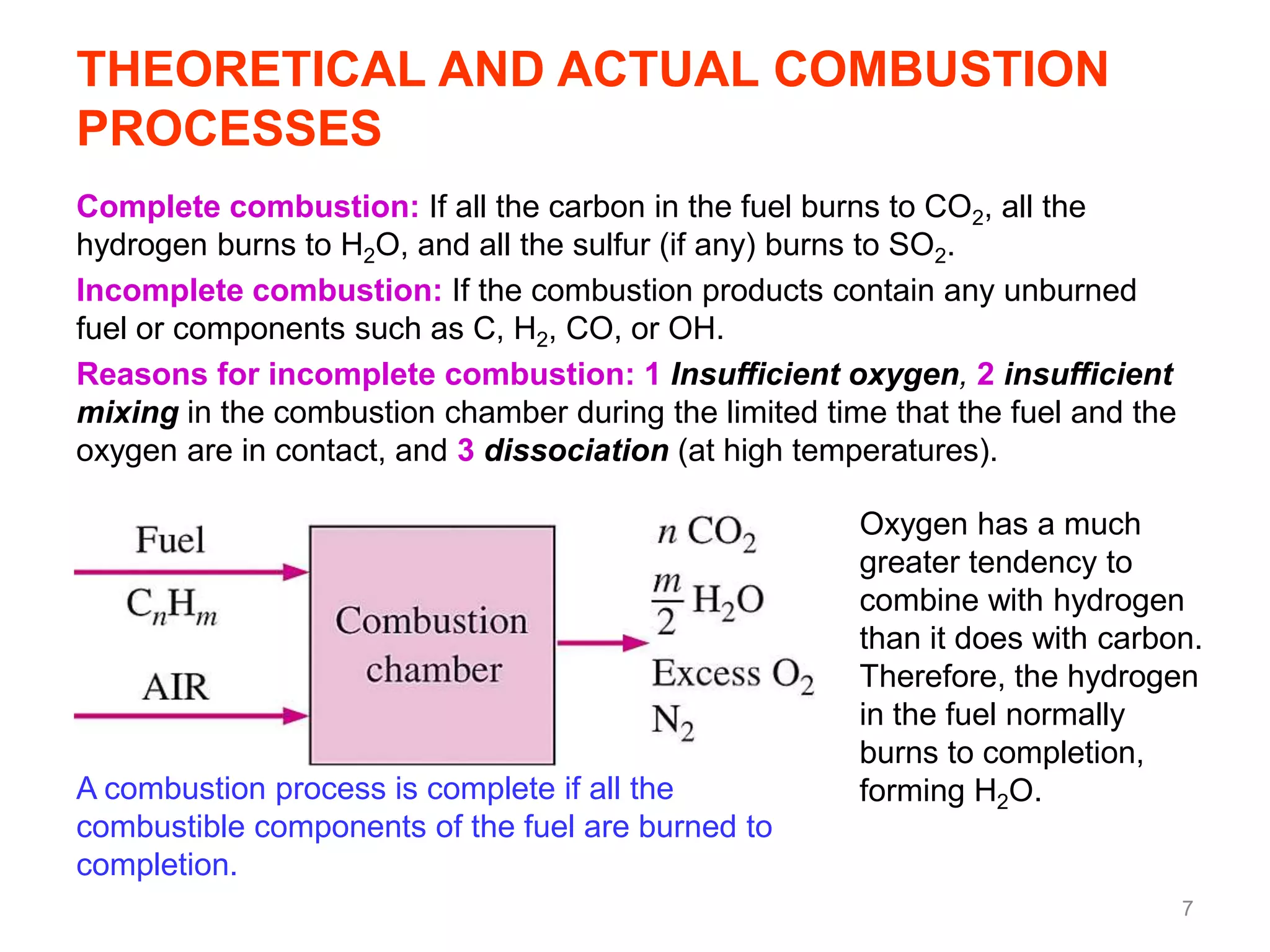
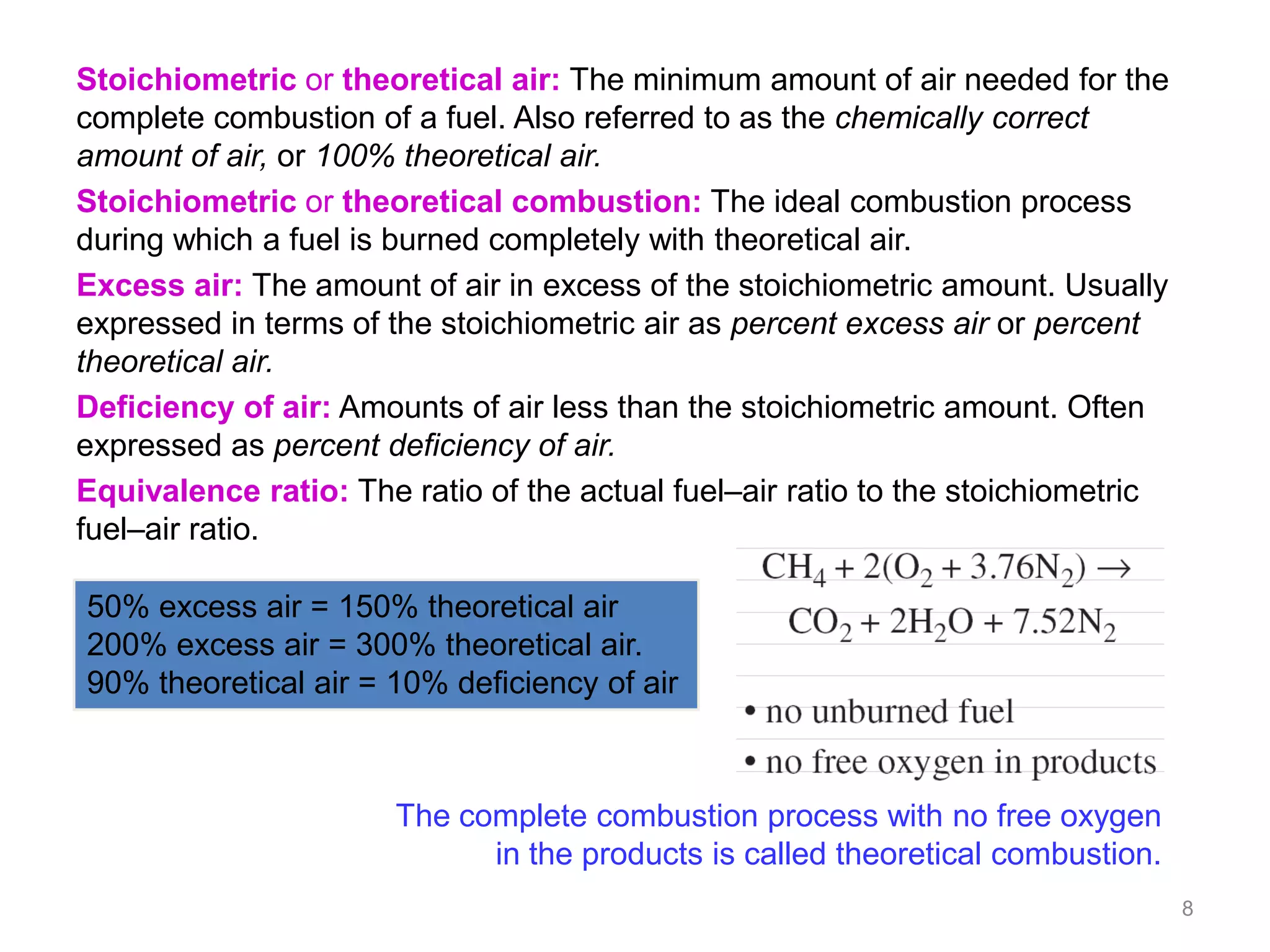
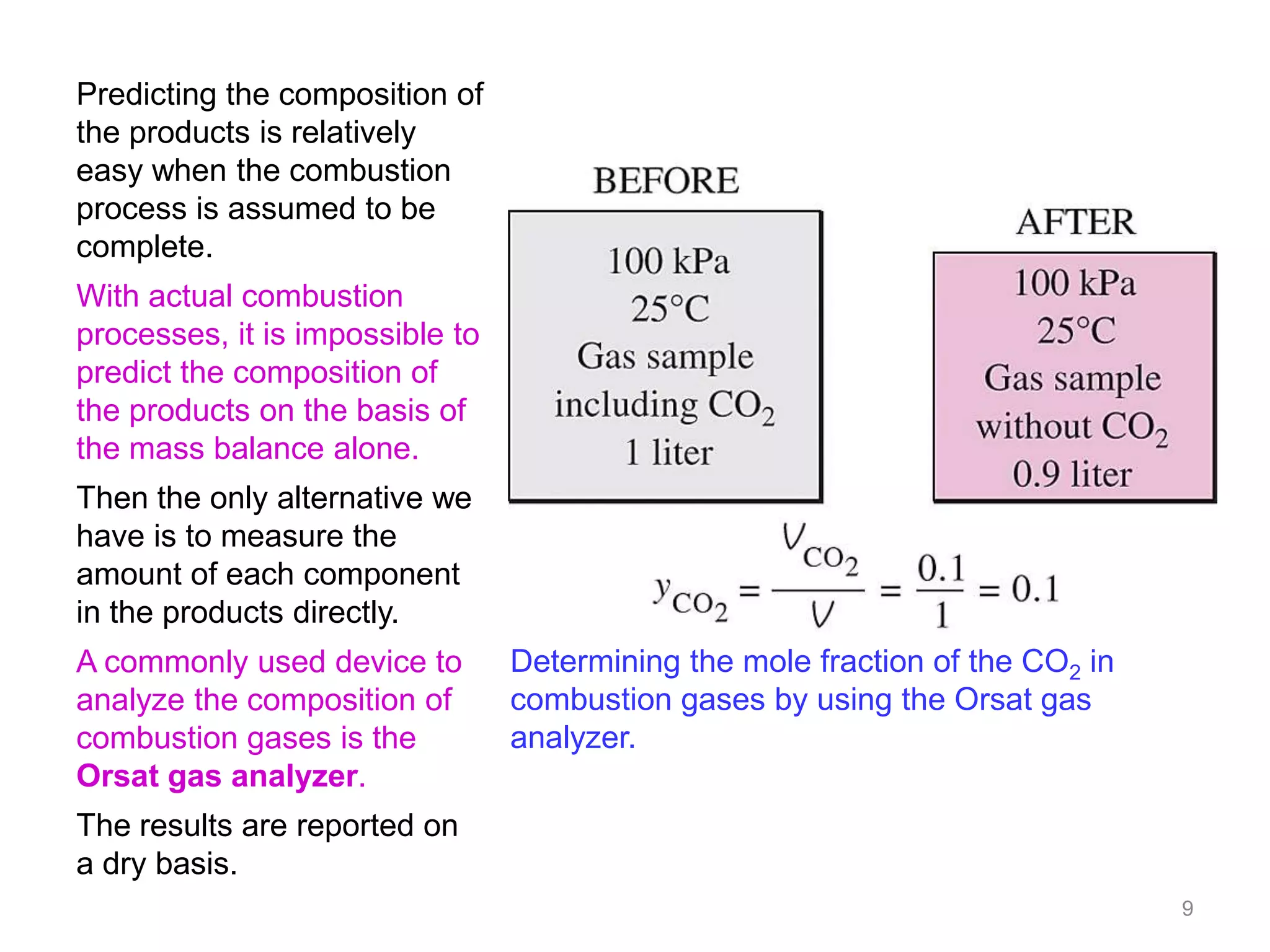
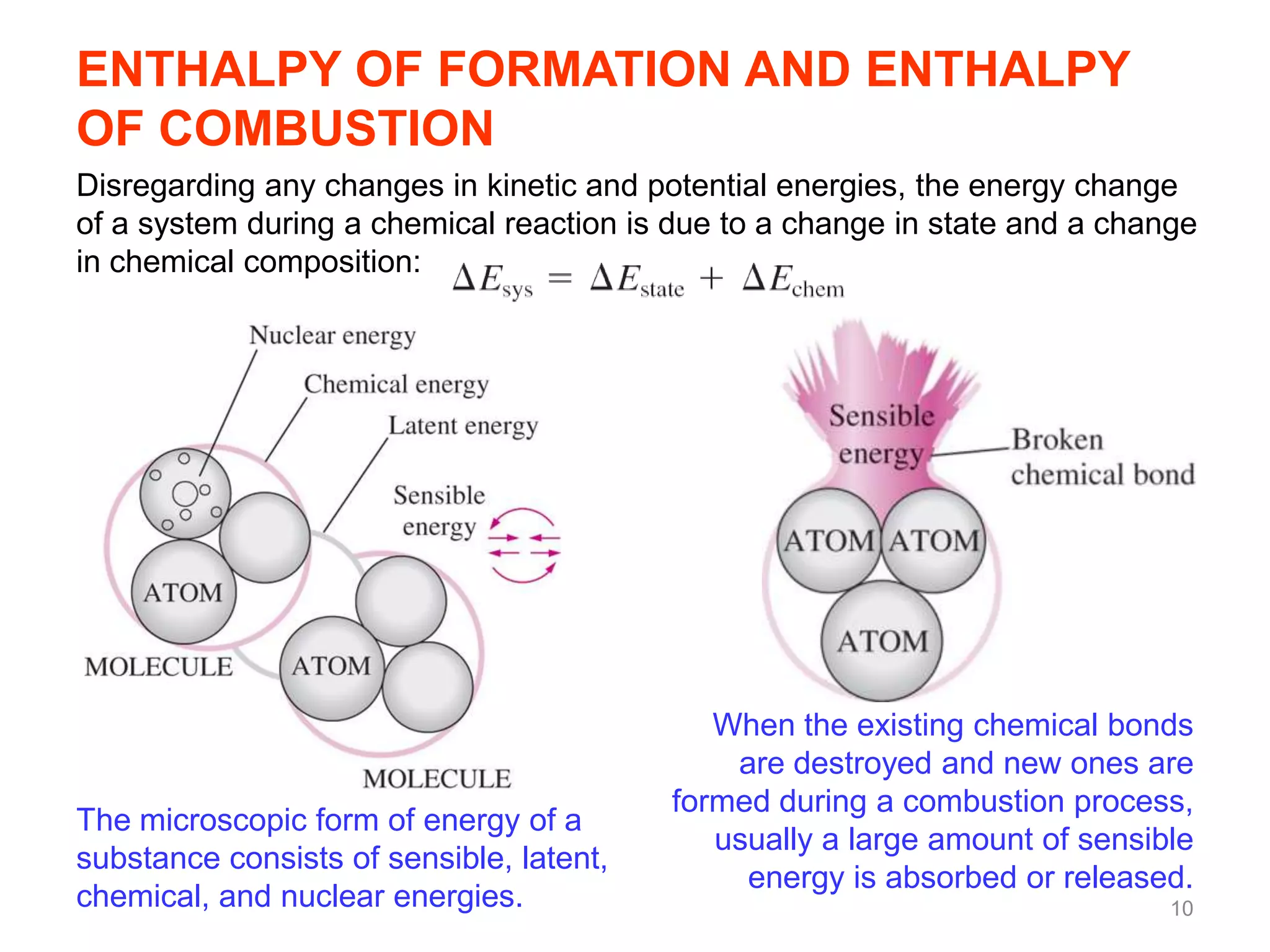
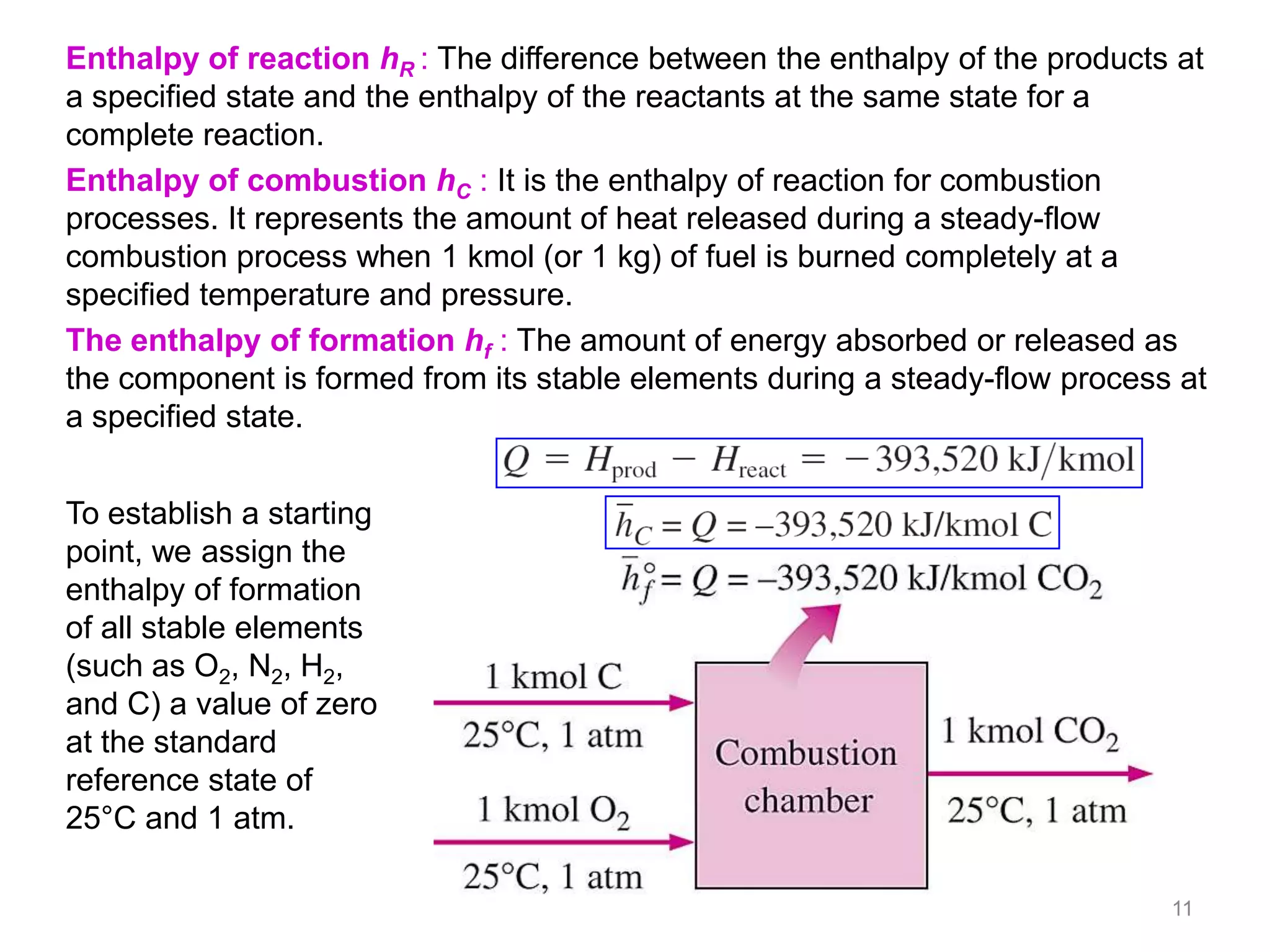
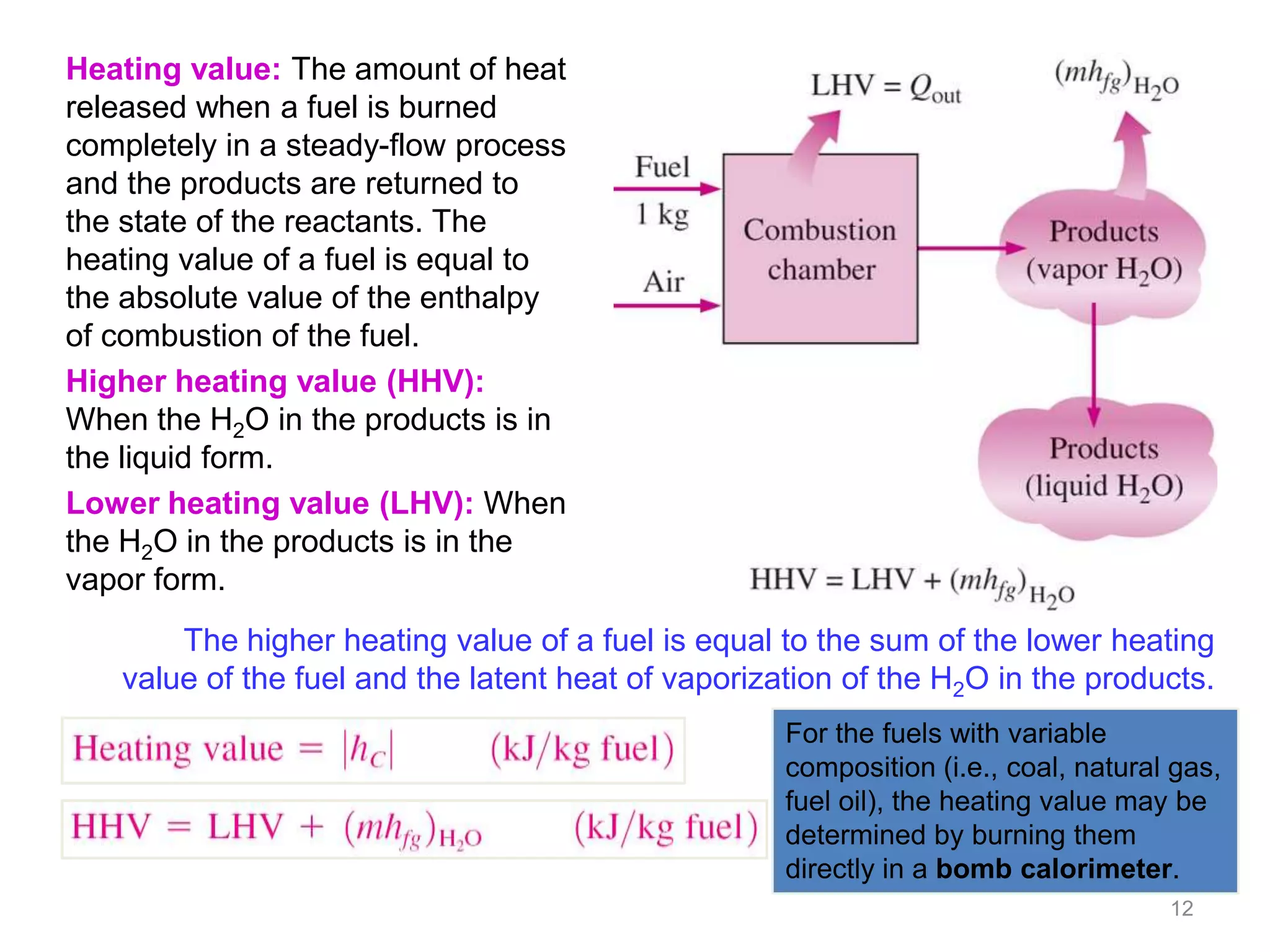
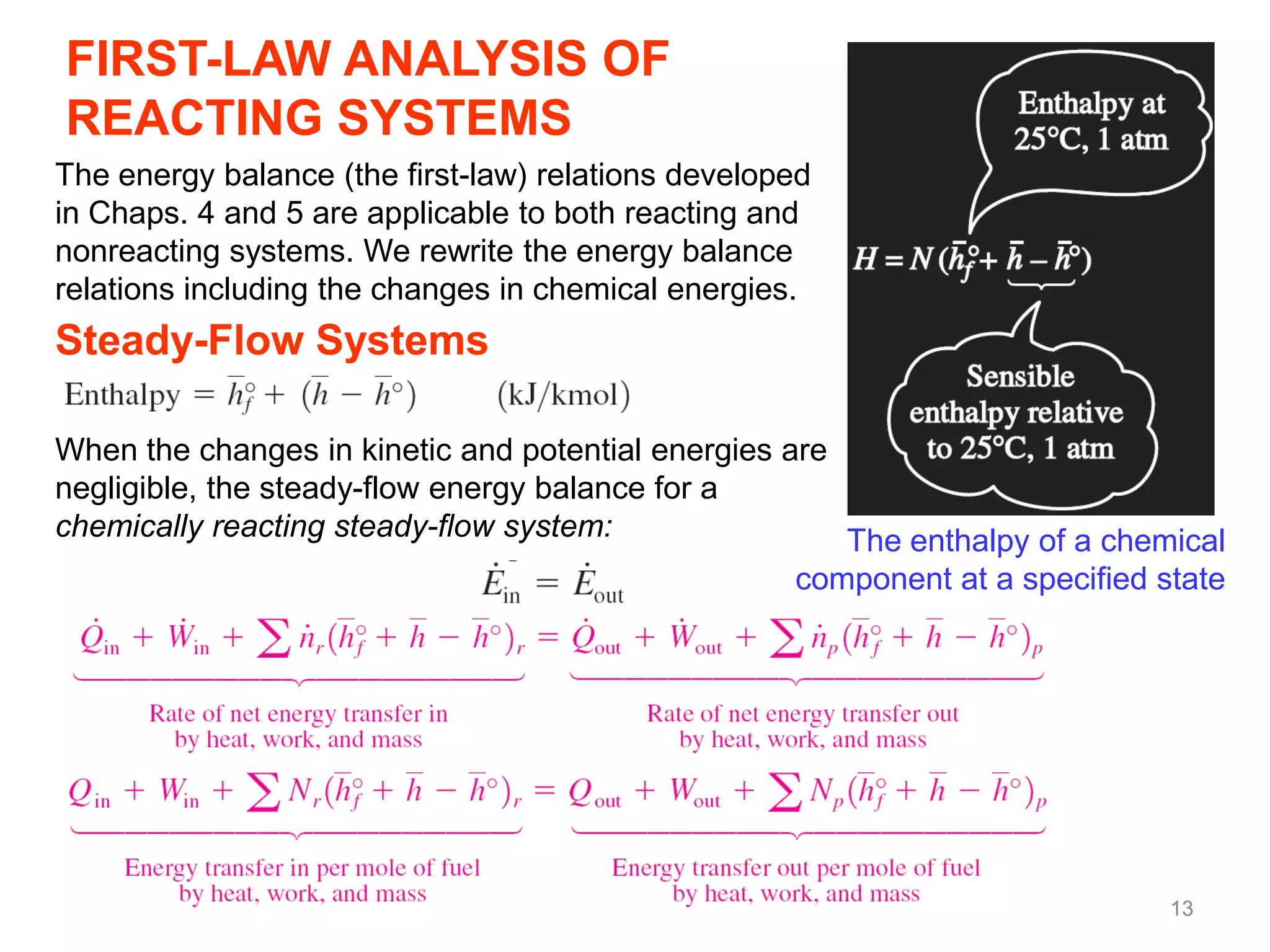
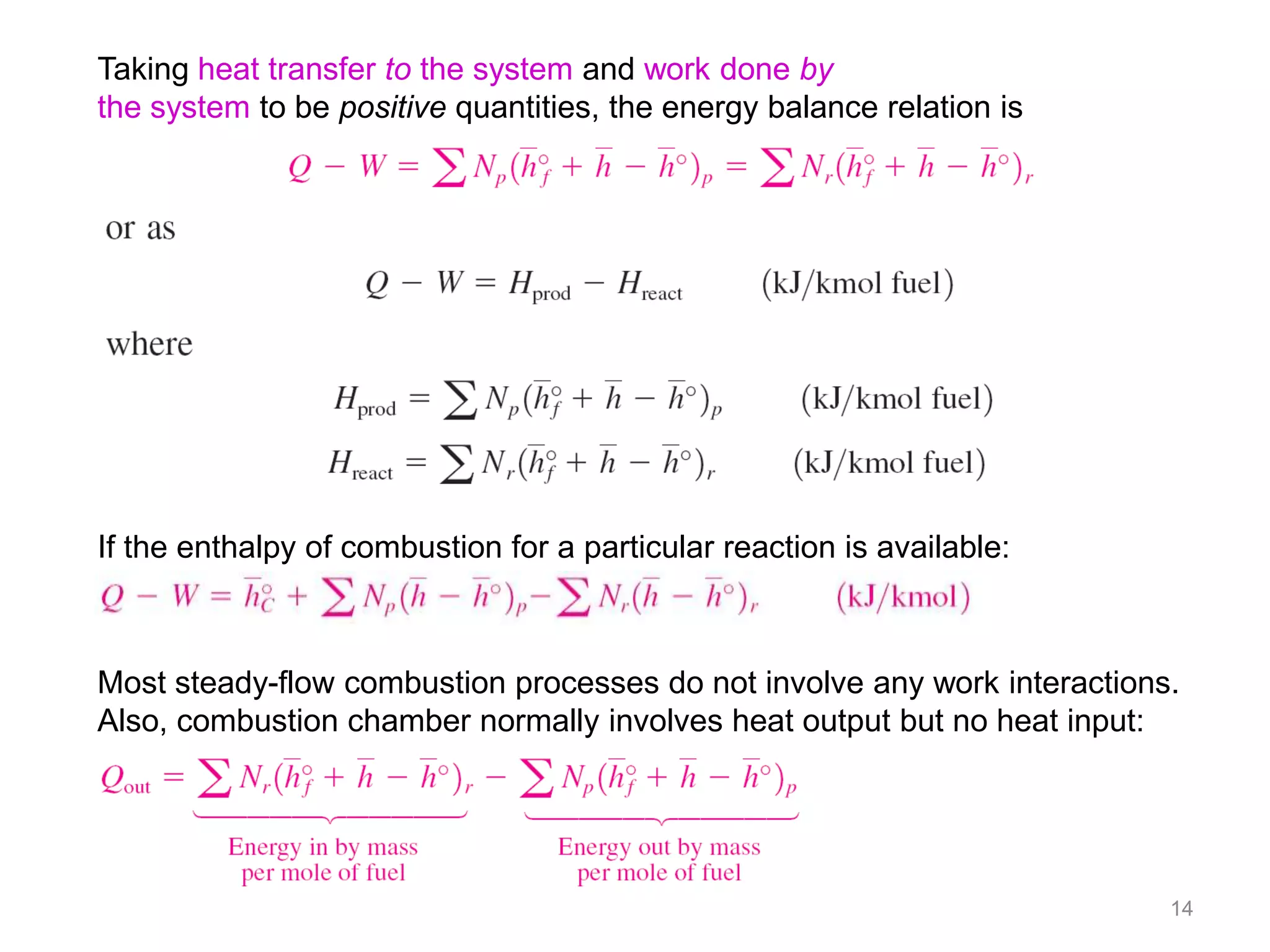
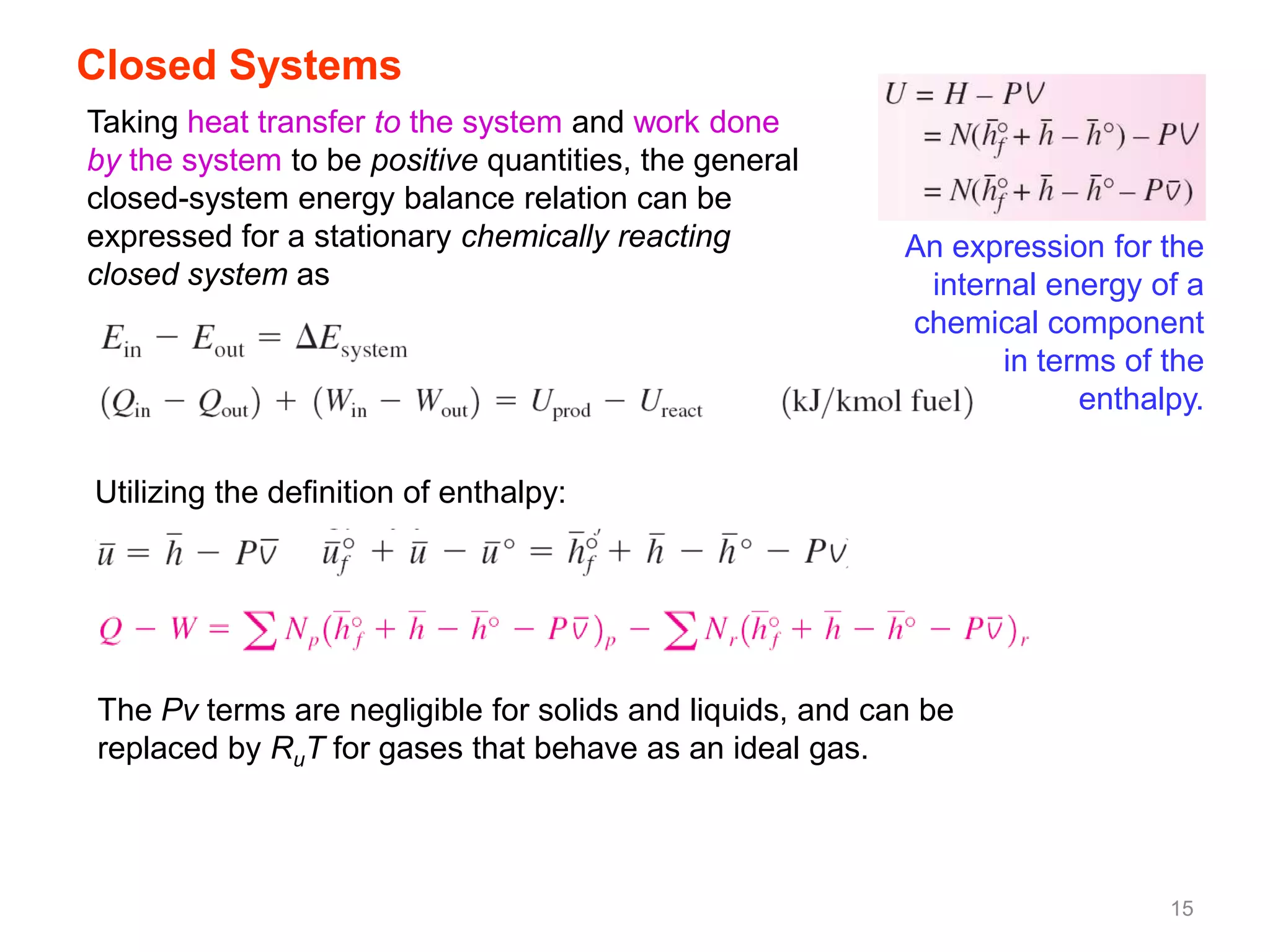
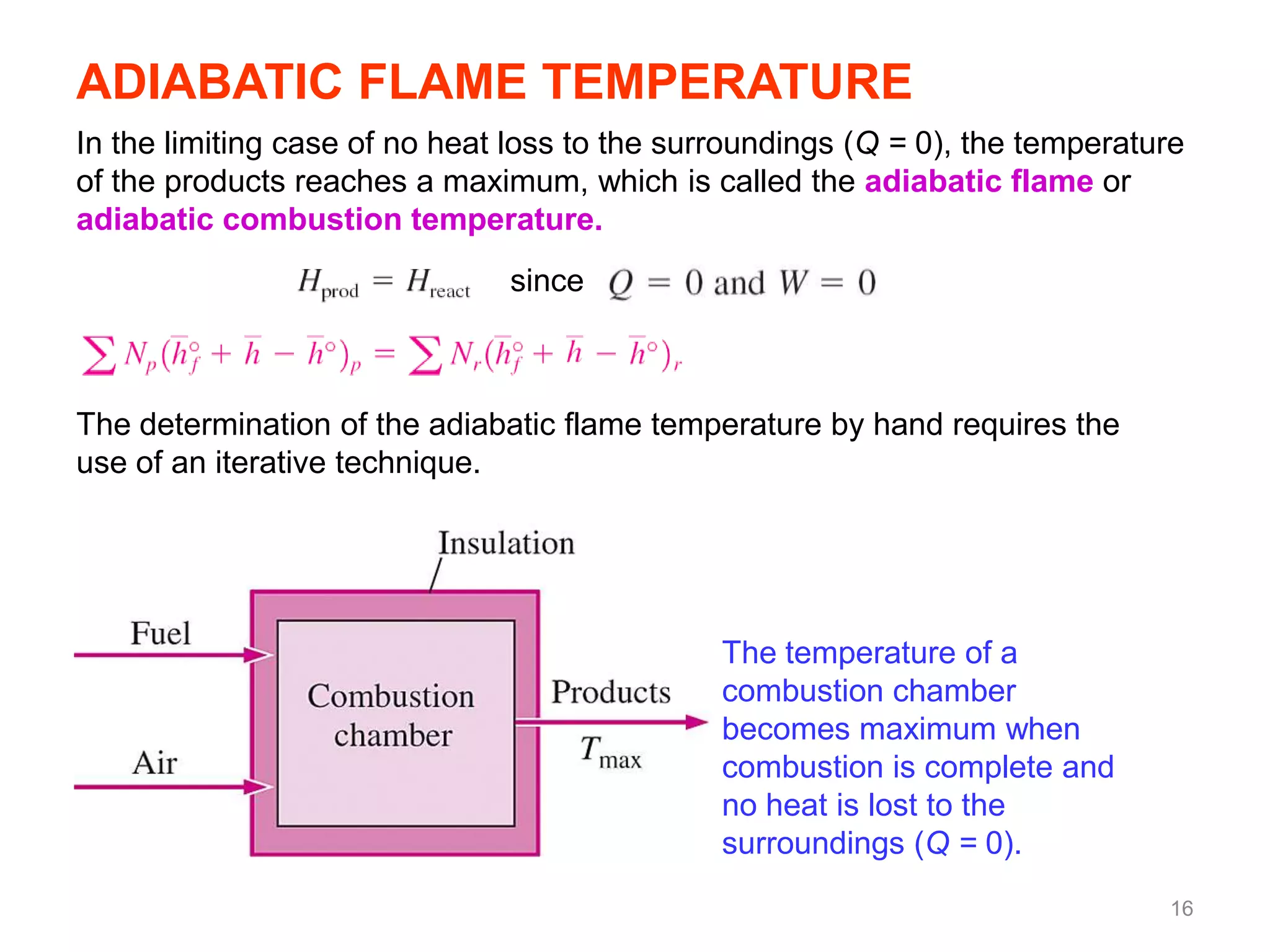
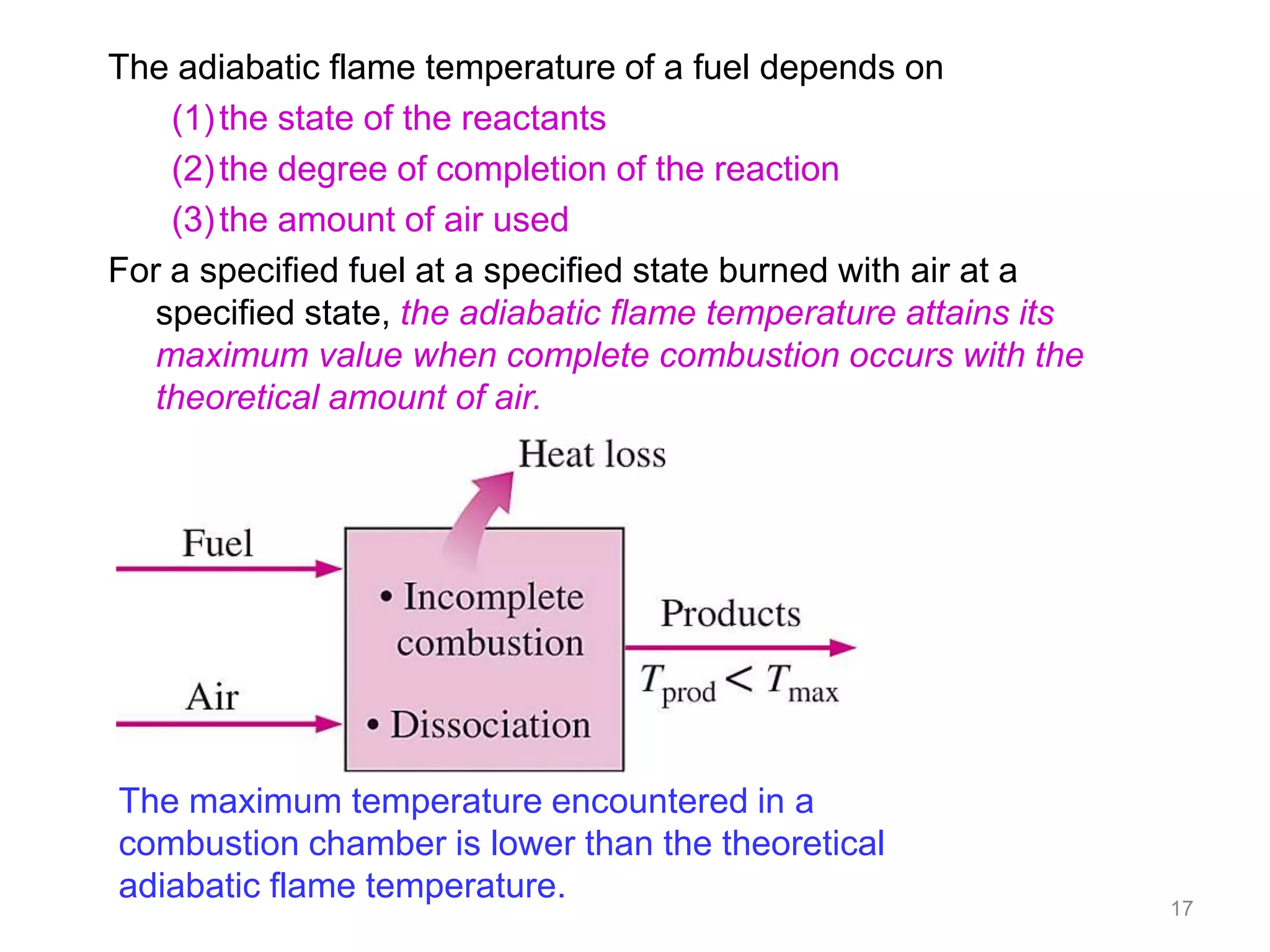
This chapter discusses fuels and combustion processes. It defines key terms like fuel, air-fuel ratio, heating values, enthalpy of combustion. It explains the concepts of theoretical and actual combustion, stoichiometric air and excess air. It also discusses first law analysis of reacting systems for both steady flow and closed systems. The chapter concludes with the concept of adiabatic flame temperature which is the maximum temperature reached during combustion if no heat is lost to the surroundings. Several examples are provided to illustrate calculations of air-fuel ratio, enthalpy of combustion and adiabatic flame temperature for different fuel-air mixtures.