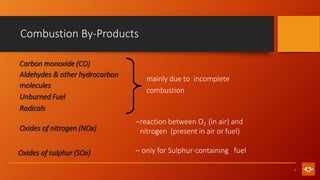
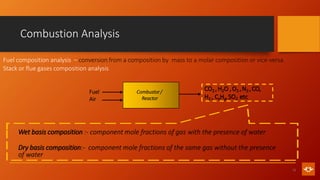
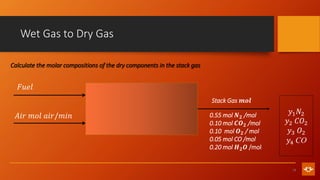
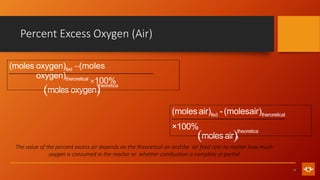
1. The document discusses various combustion processes including stoichiometric, excess air/oxygen, and incomplete combustion. It defines key terms and concepts.
2. Fossil fuel combustion is described as the chemical reaction between hydrogen and carbon in fuels with oxygen, releasing heat and forming mainly CO2 and H2O.
3. Categories of combustion processes are defined as complete, excess air/fuel, and incomplete combustion. Stoichiometric combustion involves the theoretical minimum amounts of fuel and air for complete combustion.