Chapter 3 (law of conservation of mass & and 1st law)
•Download as PPT, PDF•
0 likes•268 views
ppt 3
Report
Share
Report
Share
Recommended
Fugacity & fugacity coefficient

Introduction
Concepts of Fugacity
Effect of Temperature & pressure on Fugacity
Important relation of Fugacity Coefficient
Vapour Liquid Equilibrium for pure species
Fugacity & Fugacity coefficient: Species in solution
Reference
Recommended
Fugacity & fugacity coefficient

Introduction
Concepts of Fugacity
Effect of Temperature & pressure on Fugacity
Important relation of Fugacity Coefficient
Vapour Liquid Equilibrium for pure species
Fugacity & Fugacity coefficient: Species in solution
Reference
Derivation of Bet equation and different isotherms

This file includes BET adsorption isotherm and five types of isotherms, derivation of BET adsorption isotherms.
Fugacity & Concept of Fugacity

Chemical Thermodynamics
Fugacity & concept Fugacity
Contents:-
1. Introduction
2. Concepts of Fugacity
3. Reference
Fugacity ∝ Pressure
Fugacity =Pressure
Upload By -
Gayatri sahu
M.Sc. 1st Semester
2022
Crown ether and cryptand

BASIC DISCUSSION ABOUT THE CROWN ETHER AND CRYPTAND. INCLUDING THEIR BACKGROUND,STRUCTURE,NOMENCLATURE,CAVITY SIZE, SELECTIVITY, SYNTHESIS AND APPLICATIONS.
Molecular Weight affecting the Glass Transition temperature of Polymer

This is note about on the factors affecting glass transition temperature.
by Molecular weight in detail
Partial gibbs free energy and gibbs duhem equation

Partial gibbs free energy and gibbs duhem equation,relation between binary solution,relation between partiaL properties,PARTIAL PROPERTIES,PARTIAL PROPERTIES IN BINARY SOLUTION,RELATIONS AMONG PARTIAL PROPERTIES,Maxwell relation,Examples
Inorganic Chemistry 5th Edition Miessler Solutions Manual

FUll download : https://alibabadownload.com/product/inorganic-chemistry-5th-edition-miessler-solutions-manual/ Inorganic Chemistry 5th Edition Miessler Solutions Manual
Lect. 3 gibbs helmholtz equation, chemical potential, gibbs duhem equation

Lect. 3 gibbs helmholtz equation, chemical potential, gibbs duhem equationShri Shivaji Science College Amravati
This presentation includes Gibbs Helmholtz equation and its application. Explanation of the term Chemical potential and Gibbs Duhem equationMauvia cre ppt

This slide completely describes you about the stuff include in it and also everything about chemical engineering. Fluid Mechanics. Thermodynamics. Mass Transfer Chemical Engineering. Energy Engineering, Mass Transfer 2, Heat Transfer,
Chirality due to Heteroatoms

Chirality due to Heteroatoms: Chirality in the molecules where atoms other than carbon like N, S, Si are stereocentres
Thermodynamic I

process, Thermodynamic process,workdone, relation between pressure volume,first law of thermodynamic,need of second law,statement of second law,carnot heat engine,efficiency,numericals
More Related Content
What's hot
Derivation of Bet equation and different isotherms

This file includes BET adsorption isotherm and five types of isotherms, derivation of BET adsorption isotherms.
Fugacity & Concept of Fugacity

Chemical Thermodynamics
Fugacity & concept Fugacity
Contents:-
1. Introduction
2. Concepts of Fugacity
3. Reference
Fugacity ∝ Pressure
Fugacity =Pressure
Upload By -
Gayatri sahu
M.Sc. 1st Semester
2022
Crown ether and cryptand

BASIC DISCUSSION ABOUT THE CROWN ETHER AND CRYPTAND. INCLUDING THEIR BACKGROUND,STRUCTURE,NOMENCLATURE,CAVITY SIZE, SELECTIVITY, SYNTHESIS AND APPLICATIONS.
Molecular Weight affecting the Glass Transition temperature of Polymer

This is note about on the factors affecting glass transition temperature.
by Molecular weight in detail
Partial gibbs free energy and gibbs duhem equation

Partial gibbs free energy and gibbs duhem equation,relation between binary solution,relation between partiaL properties,PARTIAL PROPERTIES,PARTIAL PROPERTIES IN BINARY SOLUTION,RELATIONS AMONG PARTIAL PROPERTIES,Maxwell relation,Examples
Inorganic Chemistry 5th Edition Miessler Solutions Manual

FUll download : https://alibabadownload.com/product/inorganic-chemistry-5th-edition-miessler-solutions-manual/ Inorganic Chemistry 5th Edition Miessler Solutions Manual
Lect. 3 gibbs helmholtz equation, chemical potential, gibbs duhem equation

Lect. 3 gibbs helmholtz equation, chemical potential, gibbs duhem equationShri Shivaji Science College Amravati
This presentation includes Gibbs Helmholtz equation and its application. Explanation of the term Chemical potential and Gibbs Duhem equationMauvia cre ppt

This slide completely describes you about the stuff include in it and also everything about chemical engineering. Fluid Mechanics. Thermodynamics. Mass Transfer Chemical Engineering. Energy Engineering, Mass Transfer 2, Heat Transfer,
Chirality due to Heteroatoms

Chirality due to Heteroatoms: Chirality in the molecules where atoms other than carbon like N, S, Si are stereocentres
What's hot (20)
Chem II - Real Gases: Van der Waals (Liquids and Solids)

Chem II - Real Gases: Van der Waals (Liquids and Solids)
Derivation of Bet equation and different isotherms

Derivation of Bet equation and different isotherms
Molecular Weight affecting the Glass Transition temperature of Polymer

Molecular Weight affecting the Glass Transition temperature of Polymer
Partial gibbs free energy and gibbs duhem equation

Partial gibbs free energy and gibbs duhem equation
Inorganic Chemistry 5th Edition Miessler Solutions Manual

Inorganic Chemistry 5th Edition Miessler Solutions Manual
Lect. 3 gibbs helmholtz equation, chemical potential, gibbs duhem equation

Lect. 3 gibbs helmholtz equation, chemical potential, gibbs duhem equation
Similar to Chapter 3 (law of conservation of mass & and 1st law)
Thermodynamic I

process, Thermodynamic process,workdone, relation between pressure volume,first law of thermodynamic,need of second law,statement of second law,carnot heat engine,efficiency,numericals
Unit 2: BASIC MECHANICAL ENGINEERING by varun pratap singh

Free Download Link (Copy URL):
https://sites.google.com/view/varunpratapsingh/teaching-engagements
UNIT-2:
Zeroth law: Zeroth law, Different temperature scales and temperature measurement
First law: First law of thermodynamics. Processes - flow and non-flow, Control volume, Flow work and non-flow work, Steady flow energy equation, Unsteady flow systems and their analysis.
Second law: Limitations of first law of thermodynamics, Essence of second law, Thermal reservoir, Heat engines. COP of heat pump and refrigerator. Statements of the second law and their equivalence, Carnot cycle, Carnot theorem, Thermodynamic temperature scale, Clausius inequality. Concept of entropy.
Chemical Thermodynamics

Basic Terminology,Heat, energy and work, Internal Energy (E or U),First Law of Thermodynamics, Enthalpy,Molar heat capacity, Heat capacity,Specific heat capacity,Enthalpies of Reactions,Hess’s Law of constant heat summation,Born–Haber Cycle,Lattice energy,Second law of thermodynamics, Gibbs free energy(ΔG),Bond Energies,Efficiency of a heat engine
IB Chemistry on Energetics experiment, Thermodynamics and Hess's Law

IB Chemistry on Energetics experiment, Thermodynamics and Hess's Law
Similar to Chapter 3 (law of conservation of mass & and 1st law) (20)
Unit 2: BASIC MECHANICAL ENGINEERING by varun pratap singh

Unit 2: BASIC MECHANICAL ENGINEERING by varun pratap singh
IB Chemistry on Energetics experiment, Thermodynamics and Hess's Law

IB Chemistry on Energetics experiment, Thermodynamics and Hess's Law
More from Yuri Melliza
More from Yuri Melliza (20)
Module 6 (ideal or perfect gas and gas mixture) 2021 2022

Module 6 (ideal or perfect gas and gas mixture) 2021 2022
Module 1 (terms and definition & properties of fluids)2021 2022

Module 1 (terms and definition & properties of fluids)2021 2022
Recently uploaded
Democratizing Fuzzing at Scale by Abhishek Arya

Presented at NUS: Fuzzing and Software Security Summer School 2024
This keynote talks about the democratization of fuzzing at scale, highlighting the collaboration between open source communities, academia, and industry to advance the field of fuzzing. It delves into the history of fuzzing, the development of scalable fuzzing platforms, and the empowerment of community-driven research. The talk will further discuss recent advancements leveraging AI/ML and offer insights into the future evolution of the fuzzing landscape.
Immunizing Image Classifiers Against Localized Adversary Attacks

This paper addresses the vulnerability of deep learning models, particularly convolutional neural networks
(CNN)s, to adversarial attacks and presents a proactive training technique designed to counter them. We
introduce a novel volumization algorithm, which transforms 2D images into 3D volumetric representations.
When combined with 3D convolution and deep curriculum learning optimization (CLO), itsignificantly improves
the immunity of models against localized universal attacks by up to 40%. We evaluate our proposed approach
using contemporary CNN architectures and the modified Canadian Institute for Advanced Research (CIFAR-10
and CIFAR-100) and ImageNet Large Scale Visual Recognition Challenge (ILSVRC12) datasets, showcasing
accuracy improvements over previous techniques. The results indicate that the combination of the volumetric
input and curriculum learning holds significant promise for mitigating adversarial attacks without necessitating
adversary training.
Student information management system project report ii.pdf

Our project explains about the student management. This project mainly explains the various actions related to student details. This project shows some ease in adding, editing and deleting the student details. It also provides a less time consuming process for viewing, adding, editing and deleting the marks of the students.
Halogenation process of chemical process industries

This presentation is about nitration process of industries, unit processes of chemical engineering.
Cosmetic shop management system project report.pdf

Buying new cosmetic products is difficult. It can even be scary for those who have sensitive skin and are prone to skin trouble. The information needed to alleviate this problem is on the back of each product, but it's thought to interpret those ingredient lists unless you have a background in chemistry.
Instead of buying and hoping for the best, we can use data science to help us predict which products may be good fits for us. It includes various function programs to do the above mentioned tasks.
Data file handling has been effectively used in the program.
The automated cosmetic shop management system should deal with the automation of general workflow and administration process of the shop. The main processes of the system focus on customer's request where the system is able to search the most appropriate products and deliver it to the customers. It should help the employees to quickly identify the list of cosmetic product that have reached the minimum quantity and also keep a track of expired date for each cosmetic product. It should help the employees to find the rack number in which the product is placed.It is also Faster and more efficient way.
Standard Reomte Control Interface - Neometrix

About
Indigenized remote control interface card suitable for MAFI system CCR equipment. Compatible for IDM8000 CCR. Backplane mounted serial and TCP/Ethernet communication module for CCR remote access. IDM 8000 CCR remote control on serial and TCP protocol.
• Remote control: Parallel or serial interface.
• Compatible with MAFI CCR system.
• Compatible with IDM8000 CCR.
• Compatible with Backplane mount serial communication.
• Compatible with commercial and Defence aviation CCR system.
• Remote control system for accessing CCR and allied system over serial or TCP.
• Indigenized local Support/presence in India.
• Easy in configuration using DIP switches.
Technical Specifications
Indigenized remote control interface card suitable for MAFI system CCR equipment. Compatible for IDM8000 CCR. Backplane mounted serial and TCP/Ethernet communication module for CCR remote access. IDM 8000 CCR remote control on serial and TCP protocol.
Key Features
Indigenized remote control interface card suitable for MAFI system CCR equipment. Compatible for IDM8000 CCR. Backplane mounted serial and TCP/Ethernet communication module for CCR remote access. IDM 8000 CCR remote control on serial and TCP protocol.
• Remote control: Parallel or serial interface
• Compatible with MAFI CCR system
• Copatiable with IDM8000 CCR
• Compatible with Backplane mount serial communication.
• Compatible with commercial and Defence aviation CCR system.
• Remote control system for accessing CCR and allied system over serial or TCP.
• Indigenized local Support/presence in India.
Application
• Remote control: Parallel or serial interface.
• Compatible with MAFI CCR system.
• Compatible with IDM8000 CCR.
• Compatible with Backplane mount serial communication.
• Compatible with commercial and Defence aviation CCR system.
• Remote control system for accessing CCR and allied system over serial or TCP.
• Indigenized local Support/presence in India.
• Easy in configuration using DIP switches.
Sachpazis:Terzaghi Bearing Capacity Estimation in simple terms with Calculati...

Terzaghi's soil bearing capacity theory, developed by Karl Terzaghi, is a fundamental principle in geotechnical engineering used to determine the bearing capacity of shallow foundations. This theory provides a method to calculate the ultimate bearing capacity of soil, which is the maximum load per unit area that the soil can support without undergoing shear failure. The Calculation HTML Code included.
AKS UNIVERSITY Satna Final Year Project By OM Hardaha.pdf

AKS UNIVERSITY Satna Final Year Project By OM Hardaha.
Thank me later.
samsarthak31@gmail.com
Courier management system project report.pdf

It is now-a-days very important for the people to send or receive articles like imported furniture, electronic items, gifts, business goods and the like. People depend vastly on different transport systems which mostly use the manual way of receiving and delivering the articles. There is no way to track the articles till they are received and there is no way to let the customer know what happened in transit, once he booked some articles. In such a situation, we need a system which completely computerizes the cargo activities including time to time tracking of the articles sent. This need is fulfilled by Courier Management System software which is online software for the cargo management people that enables them to receive the goods from a source and send them to a required destination and track their status from time to time.
Architectural Portfolio Sean Lockwood

This portfolio contains selected projects I completed during my undergraduate studies. 2018 - 2023
HYDROPOWER - Hydroelectric power generation

Overview of the fundamental roles in Hydropower generation and the components involved in wider Electrical Engineering.
This paper presents the design and construction of hydroelectric dams from the hydrologist’s survey of the valley before construction, all aspects and involved disciplines, fluid dynamics, structural engineering, generation and mains frequency regulation to the very transmission of power through the network in the United Kingdom.
Author: Robbie Edward Sayers
Collaborators and co editors: Charlie Sims and Connor Healey.
(C) 2024 Robbie E. Sayers
Top 10 Oil and Gas Projects in Saudi Arabia 2024.pdf

Saudi Arabia stands as a titan in the global energy landscape, renowned for its abundant oil and gas resources. It's the largest exporter of petroleum and holds some of the world's most significant reserves. Let's delve into the top 10 oil and gas projects shaping Saudi Arabia's energy future in 2024.
Nuclear Power Economics and Structuring 2024

Title: Nuclear Power Economics and Structuring - 2024 Edition
Produced by: World Nuclear Association Published: April 2024
Report No. 2024/001
© 2024 World Nuclear Association.
Registered in England and Wales, company number 01215741
This report reflects the views
of industry experts but does not
necessarily represent those
of World Nuclear Association’s
individual member organizations.
COLLEGE BUS MANAGEMENT SYSTEM PROJECT REPORT.pdf

The College Bus Management system is completely developed by Visual Basic .NET Version. The application is connect with most secured database language MS SQL Server. The application is develop by using best combination of front-end and back-end languages. The application is totally design like flat user interface. This flat user interface is more attractive user interface in 2017. The application is gives more important to the system functionality. The application is to manage the student’s details, driver’s details, bus details, bus route details, bus fees details and more. The application has only one unit for admin. The admin can manage the entire application. The admin can login into the application by using username and password of the admin. The application is develop for big and small colleges. It is more user friendly for non-computer person. Even they can easily learn how to manage the application within hours. The application is more secure by the admin. The system will give an effective output for the VB.Net and SQL Server given as input to the system. The compiled java program given as input to the system, after scanning the program will generate different reports. The application generates the report for users. The admin can view and download the report of the data. The application deliver the excel format reports. Because, excel formatted reports is very easy to understand the income and expense of the college bus. This application is mainly develop for windows operating system users. In 2017, 73% of people enterprises are using windows operating system. So the application will easily install for all the windows operating system users. The application-developed size is very low. The application consumes very low space in disk. Therefore, the user can allocate very minimum local disk space for this application.
Final project report on grocery store management system..pdf

In today’s fast-changing business environment, it’s extremely important to be able to respond to client needs in the most effective and timely manner. If your customers wish to see your business online and have instant access to your products or services.
Online Grocery Store is an e-commerce website, which retails various grocery products. This project allows viewing various products available enables registered users to purchase desired products instantly using Paytm, UPI payment processor (Instant Pay) and also can place order by using Cash on Delivery (Pay Later) option. This project provides an easy access to Administrators and Managers to view orders placed using Pay Later and Instant Pay options.
In order to develop an e-commerce website, a number of Technologies must be studied and understood. These include multi-tiered architecture, server and client-side scripting techniques, implementation technologies, programming language (such as PHP, HTML, CSS, JavaScript) and MySQL relational databases. This is a project with the objective to develop a basic website where a consumer is provided with a shopping cart website and also to know about the technologies used to develop such a website.
This document will discuss each of the underlying technologies to create and implement an e- commerce website.
Recently uploaded (20)
LIGA(E)11111111111111111111111111111111111111111.ppt

LIGA(E)11111111111111111111111111111111111111111.ppt
Immunizing Image Classifiers Against Localized Adversary Attacks

Immunizing Image Classifiers Against Localized Adversary Attacks
Student information management system project report ii.pdf

Student information management system project report ii.pdf
Halogenation process of chemical process industries

Halogenation process of chemical process industries
Cosmetic shop management system project report.pdf

Cosmetic shop management system project report.pdf
Sachpazis:Terzaghi Bearing Capacity Estimation in simple terms with Calculati...

Sachpazis:Terzaghi Bearing Capacity Estimation in simple terms with Calculati...
AKS UNIVERSITY Satna Final Year Project By OM Hardaha.pdf

AKS UNIVERSITY Satna Final Year Project By OM Hardaha.pdf
Top 10 Oil and Gas Projects in Saudi Arabia 2024.pdf

Top 10 Oil and Gas Projects in Saudi Arabia 2024.pdf
Final project report on grocery store management system..pdf

Final project report on grocery store management system..pdf
Chapter 3 (law of conservation of mass & and 1st law)
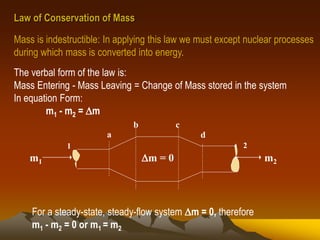
- 1. Law of Conservation of Mass Mass is indestructible: In applying this law we must except nuclear processes during which mass is converted into energy. The verbal form of the law is: Mass Entering - Mass Leaving = Change of Mass stored in the system In equation Form: m1 - m2 = m 1 2 m1 m2m = 0 a b c d For a steady-state, steady-flow system m = 0, therefore m1 - m2 = 0 or m1 = m2
- 2. For one dimensional flow, where1 = 2 = Let m1 = m2 = m Continuity Equation: υ Av Avρm Where: m - mass flw rate in kg/sec - density in kg/m3 - specific volume inm3/kg A - cross sectional area in m2 v - velocity in m/sec υυυ ρ ΑvvΑvΑ ΑvvΑρvΑρ mmm 2 22 1 11 222111 21
- 3. Zeroth Law of Thermodynamics If two bodies are in thermal equilibrium with a third body, they are in thermal equilibrium with each other, and hence their temperatures are equal. Specific Heat or Heat Capacity: It the amount of heat required to raise the temperature of a 1 kg mass of a substance 1C or 1K. tmCTmCQ m;gConsiderin CdtCdTdQ C;constantFor K-kg KJ or C-kg KJ dt dQ dT dQ C
- 4. SENSIBLE HEAT: The amount of heat per unit mass that must be transferred (added or remove) when a substance undergoes a change in temperature without a change in phase. Q = mC(t) = mC(T) where: m - mass , kg C - heat capacity or specific heat, KJ/kg-C or KJ/kg- K t - temperature in C T - temperature in K HEAT OF TRANSFORMATION: The amount of heat per unit mass that must be transferred when a substance completely undergoes a phase change without a change in temperature. Q = mL
- 5. A. Heat of Vaporization: Amount of heat that must be added to vaporize a liquid or that must be removed to condense a gas. Q = mL where L - latent heat of vaporization, KJ/kg B. Heat of Fusion : Amount of heat that must be added to melt a solid or that must be removed to freeze a liquid. Q = mL where L - latent heat of fusion, KJ/kg
- 6. THE FIRST LAW OF THERMODYNAMICS (The Law of Conservation of (Energy) “Energy can neither be created nor destroyed but can only be converted from one form to another.” Verbal Form: Energy Entering – Energy Leaving = Change of Energy stored in the system Equation Form: E1 – E2 = Es 1. First Corollary of the First Law: Application of first Law to a Closed System U Q W For a Closed System (Non FlowSystem), PV, KE and PE are negligible, therefore the changeof stored energy Es = U Q – W = U 1 Q = U + W 2
- 7. By differentiation: dQ = dU + dW 3 where: dQ Q2 – Q1 dW W2 – W1 Work of a Closed System (NonFlow) P V W = PdV P dV W = Fdx F = PA W = PAdx Adx = dV W = PdV dW = PdV From Eq. 3 dQ = dU + dW dQ = dU + PdV 4
- 8. 2. Second Corollary of the First Law: Application of First Law to an Open System System or Control volume Datum Line Q W 1 2 U1 + P1V1 + KE1 + PE1 U2 + P2V2 + KE2 + PE2 For an Open system (Steady state, Steady Flow system) Es = 0, therefore E1 – E2 = 0 or E1 = E2 or Energy Entering = Energy Leaving Z1 Z2
- 9. U1 + P1V1 + KE1 + PE1 + Q = U2 + P2V2 + KE2 + PE2 + W 1 Q = (U2 – U1) + (P2V2 – P1V1) + (KE2 – KE1) + (PE2 – PE1) + W 2 Q = U + (PV) + KE + PE + W 3 By differentiation dQ = dU + d(PV) + dKE + dPE + dW 4 But dQ Q2 – Q1 and dW W2 – W1 Enthalpy (h) h = U + PV dh = dU + d(PV) 5 dh = dU + PdV + VdP 6 But: dQ = dU + PdV dh = dQ + VdP 7 From Eq. 3 Q = h + KE + PE + W 8 dQ = dh + dKE + dPE + dW 9 dQ = dU + PdV + VdP + dKE + dPE + dW 10 dQ = dQ + VdP + dKE + dPE + dW 0 = VdP + dKE + dPE + dW dW = -VdP - dKE - dPE 11 By Integration W = - VdP - KE - PE 12
- 10. If KE = 0 and PE = 0 Q = h + W 13 W = Q - h 14 W = - VdP 15
