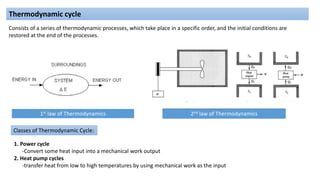
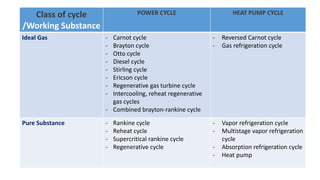
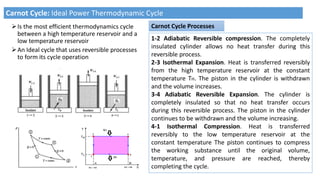
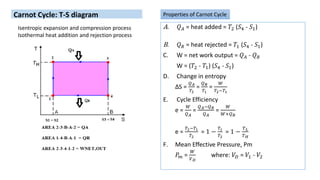
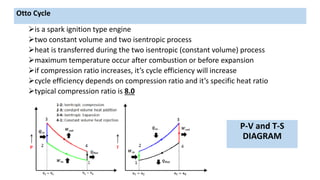
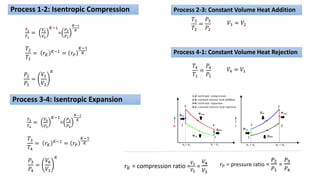
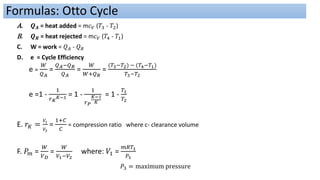
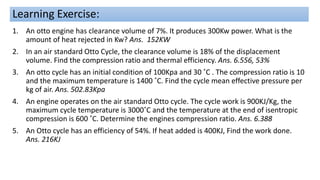
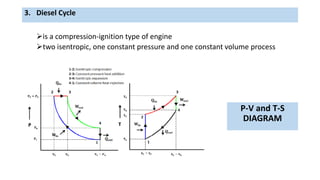
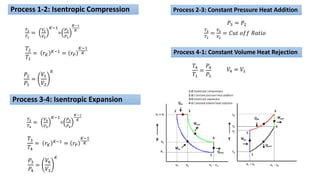
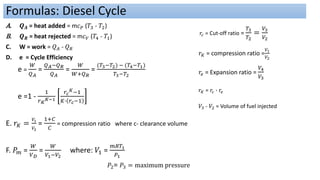
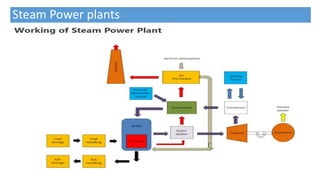
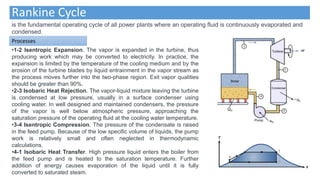
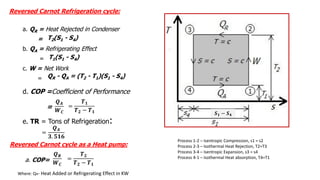
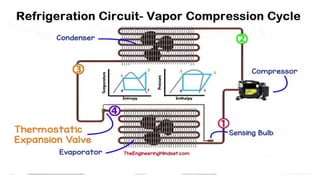
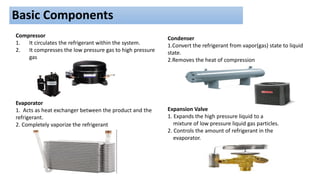
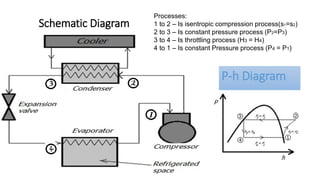
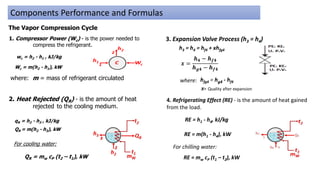
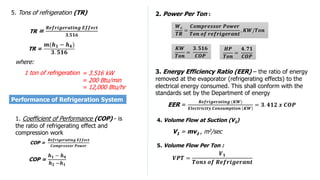
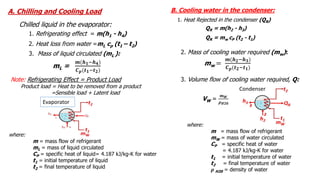
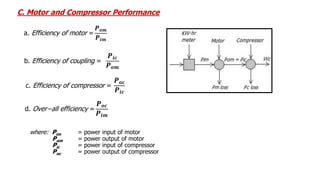
This document discusses thermodynamic cycles and steam power plants. It describes the Carnot, Otto, and Diesel cycles, outlining their key processes on P-V and T-S diagrams. It provides the basic equations and properties for each cycle. The document also lists the typical components of a steam power plant, including coal storage, coal handling, the boiler, air preheater, economizer, turbine, and generator.