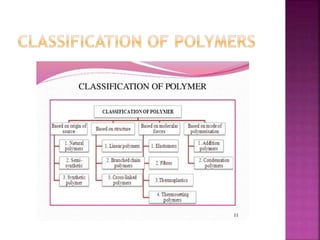
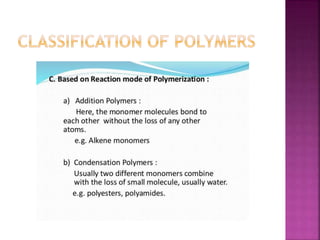
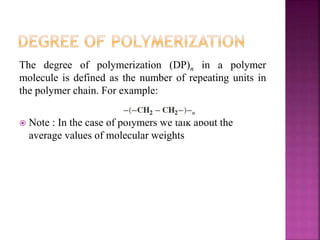
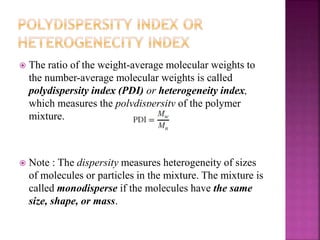
Polymers are long chain organic molecules assembled from smaller molecules called monomers. They represent an important constituent of pharmaceutical dosage forms. There are hydrophilic polymers like cellulosic polymers (methylcellulose, hydroxypropylmethylcellulose) and non-cellulosic polymers (sodium alginate, xanthan gum, carrageenan). Hydrophobic polymers include ethylcellulose and hypromellose acetate succinate. The physical and mechanical properties of polymers depend on their molecular weight, degree of polymerization, and polydispersity index. Common polymer characteristics such as solubility, viscosity range and uses in formulations are also discussed.