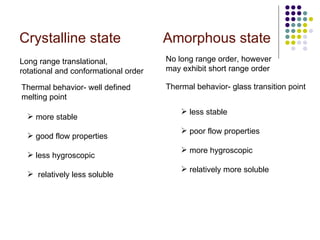
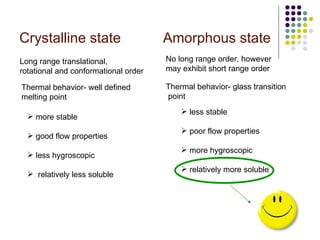
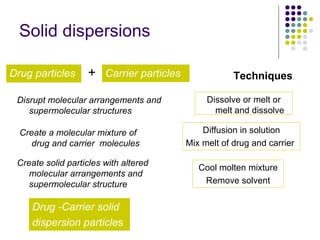
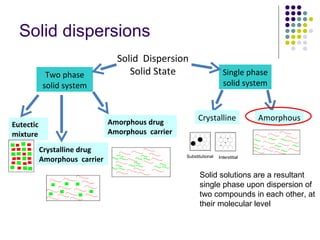
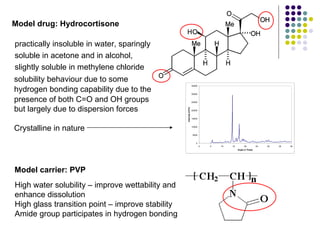
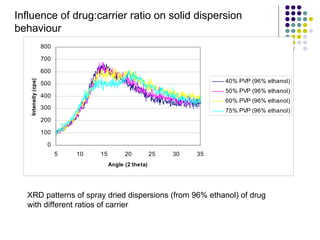
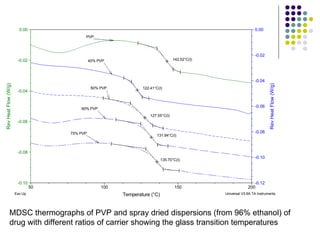
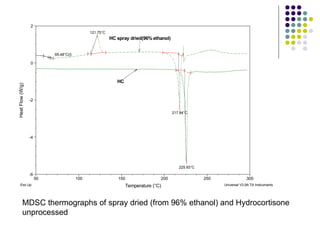
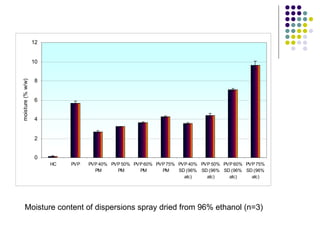
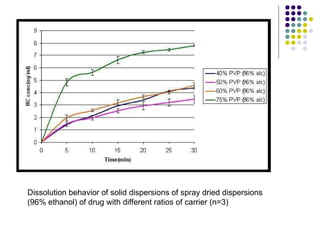
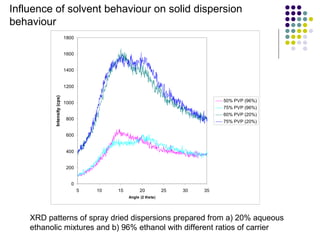
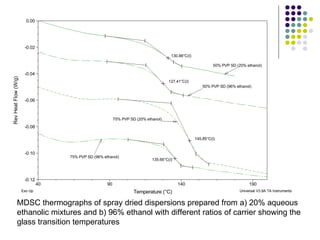
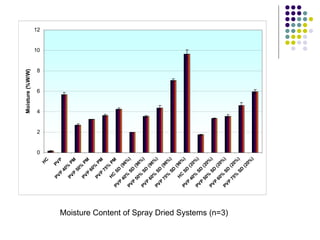
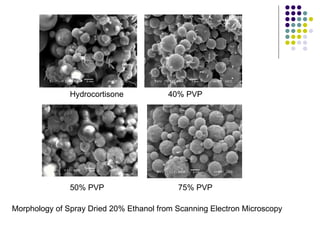
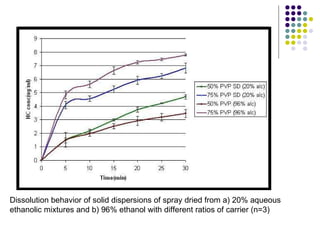
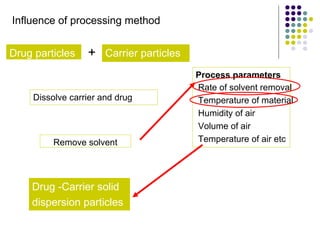
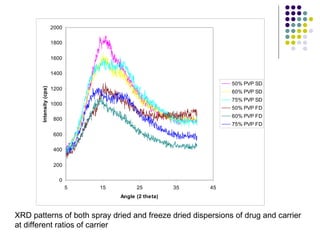
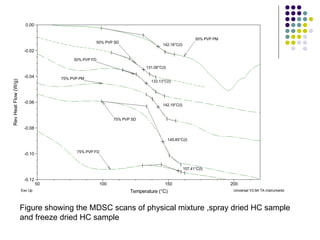
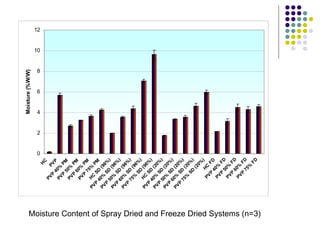
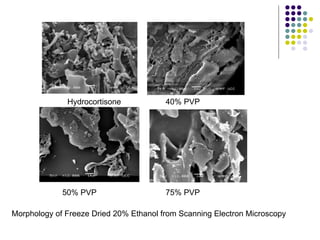
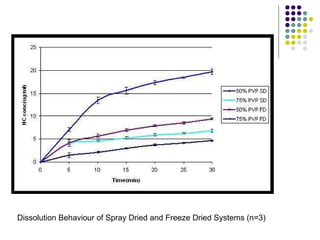
The document discusses amorphous solid dispersions and their advantages over crystalline forms. It examines the preparation and characterization of solid dispersions using hydrocortisone as a model drug and PVP as a carrier. The influence of drug to carrier ratio, solvent composition, and processing method are investigated. Spray drying and freeze drying were used to prepare dispersions from ethanol or ethanol/water solutions. Drug to carrier ratio, solvent composition, and processing method all impacted properties like glass transition temperature, moisture content, and drug dissolution rate from the solid dispersions. Further characterization and stability testing of the amorphous dispersions are recommended.