The document provides information on naming and writing formulas for various types of chemical compounds including:
- Monoatomic ions and how they are named based on their group number and charge.
- Transition metal ions and how their charge is indicated using Roman numerals.
- Polyatomic ions which contain more than one atom and have different naming conventions based on their ending.
- Binary ionic compounds and how to write their formulas by balancing the charges of the ions using subscripts.
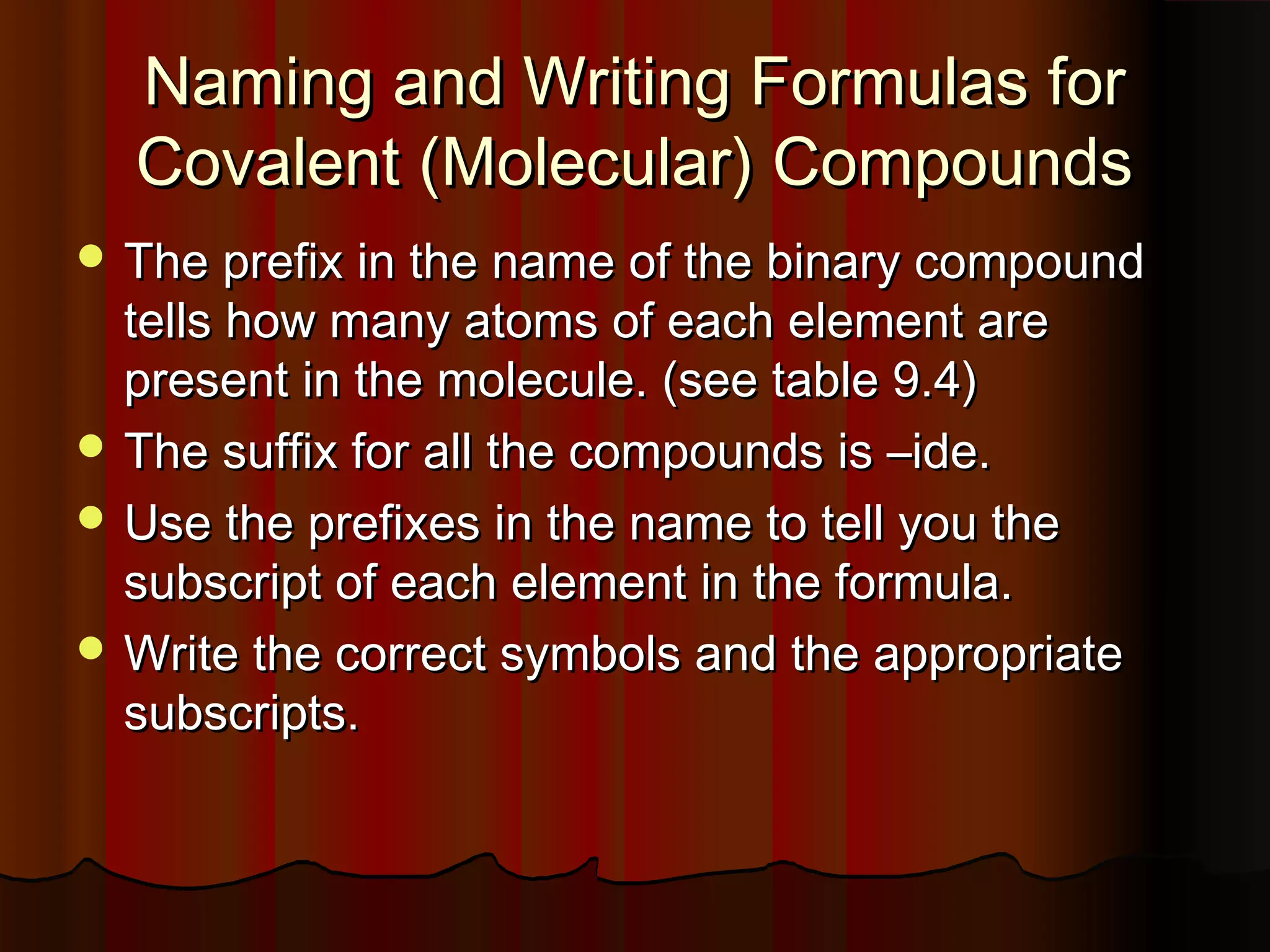
- Molecular and acidic compounds and how the prefixes and suffixes in their names relate to the number of atoms in their formulas.
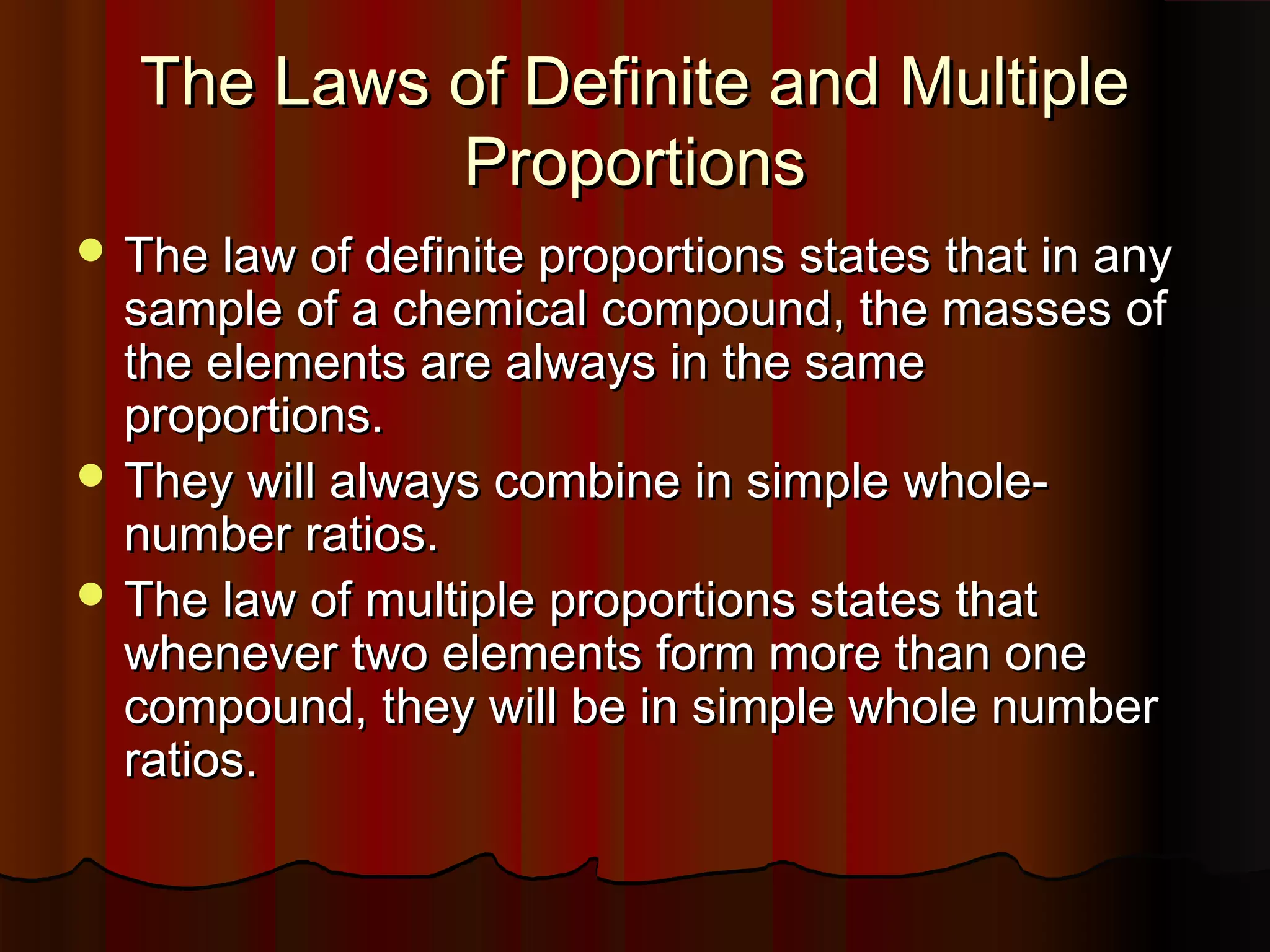
The document also discusses the laws of definite and multiple proportions and provides examples of writing word equations for