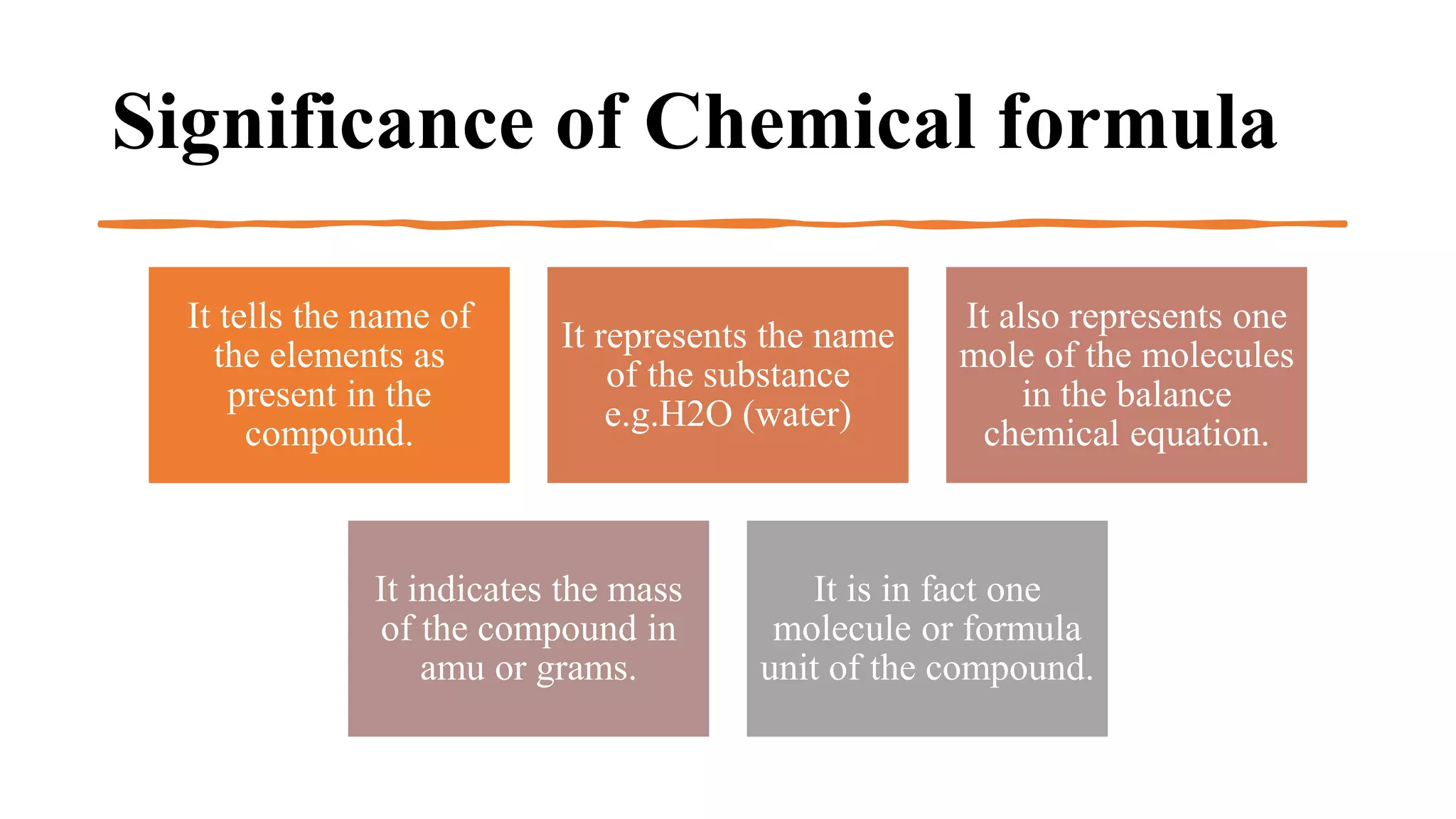
The document explains how to write a chemical formula. A chemical formula tells us the number of atoms of each element in a compound using element symbols and subscripts. It represents one mole of molecules in a balanced chemical equation and indicates the mass of the compound. To write a formula, the symbols of elements are written side-by-side in order of positive then negative ions. Valencies are written and offset or included based on if they are the same or different. Formulas also include negative radicals with their net charge representing valency.