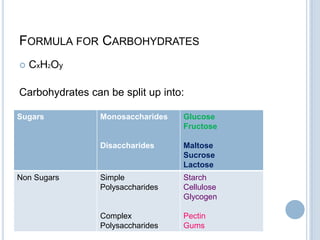
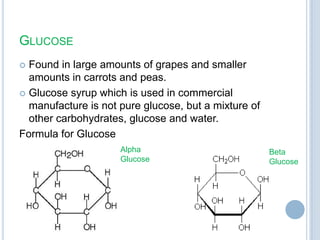
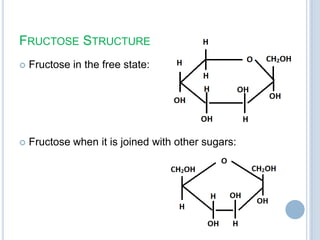
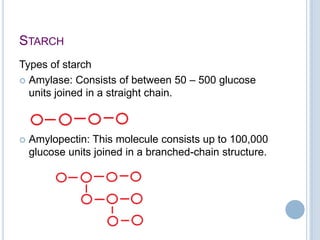
Carbohydrates can be categorized as monosaccharides, disaccharides, and polysaccharides. Glucose, fructose, and galactose are common monosaccharides that make up disaccharides like sucrose, lactose, and maltose. Starch is a storage polysaccharide made of amylose and amylopectin chains of glucose. Starch gelatinizes when heated in water. Cellulose provides fiber and is found in plant cell walls. Modified starches are used as thickeners and fat replacers in processed foods.