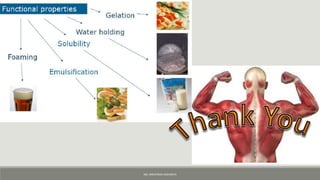
The document discusses the functional properties of proteins in foods. It begins by defining functionality as any non-nutritive property that influences an ingredient's usefulness. It then identifies three main groups of functional properties: hydration properties related to protein-water interactions like solubility and viscosity; properties related to protein-protein interactions like gelation and dough formation; and surface properties involved in emulsification and foaming. Specific examples covered include the role of proteins in viscosity, gelation, emulsions, foams, and dough formation. The document concludes by noting how extrusion and enzymatic hydrolysis can alter soy protein properties.