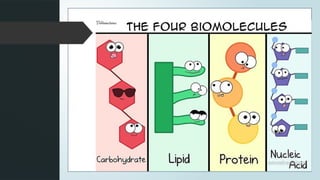
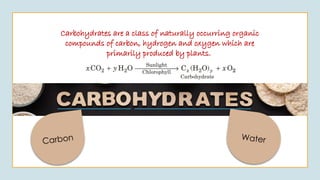
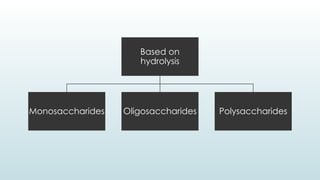
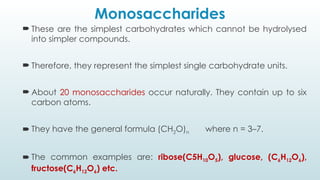
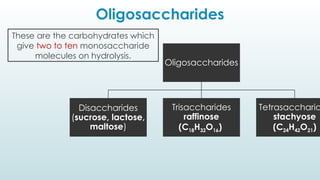
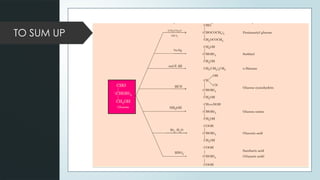
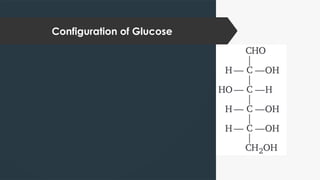
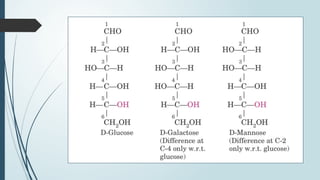
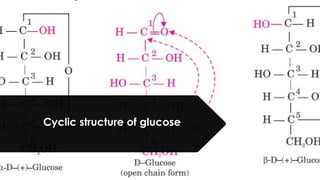
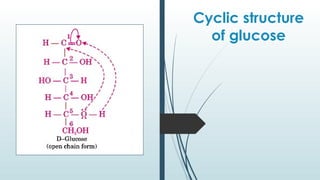
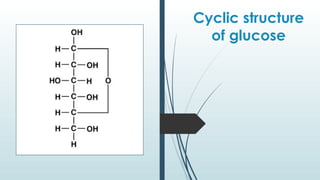
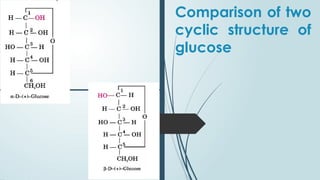
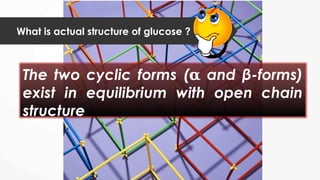
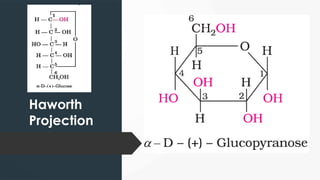
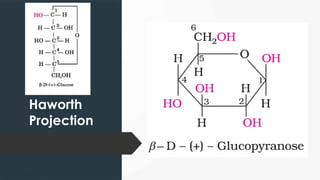
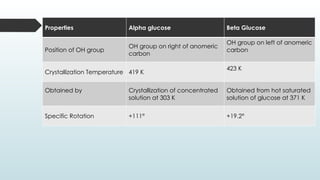
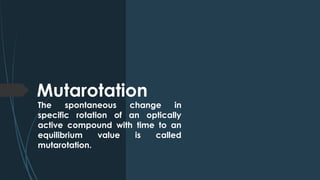
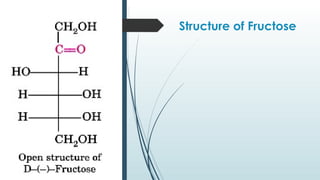
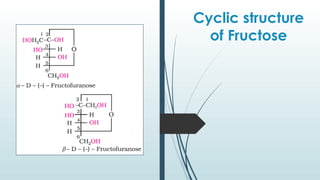
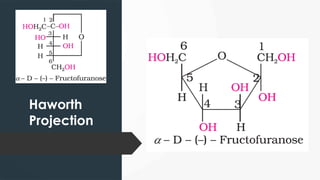
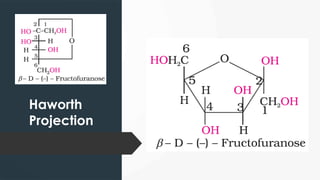
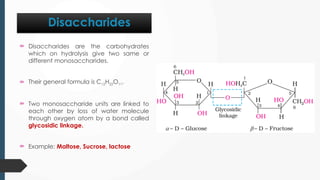
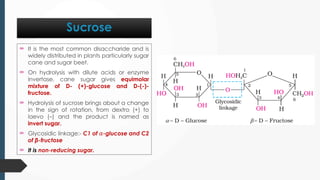
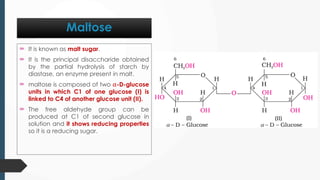
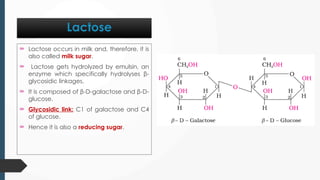
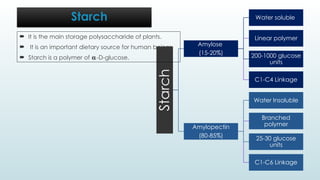
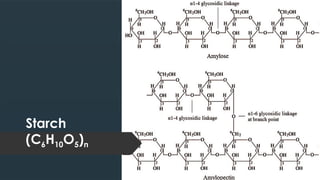
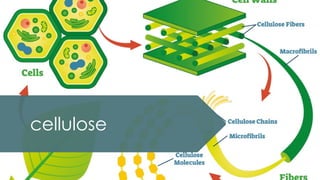
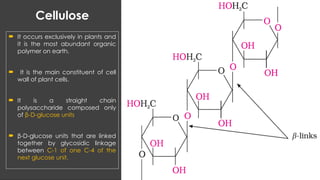
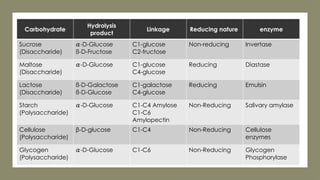
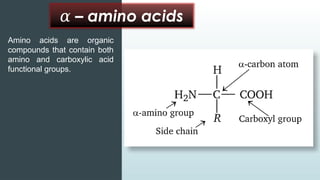
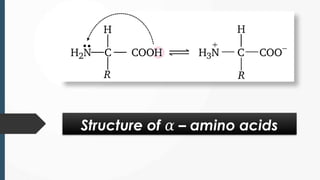
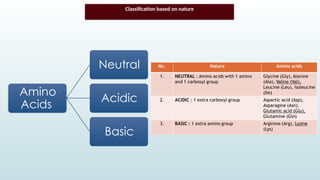
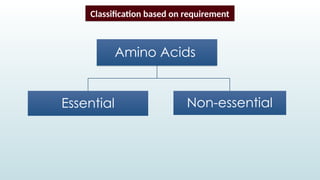
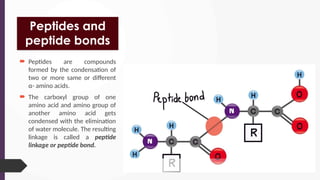
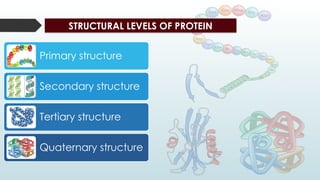
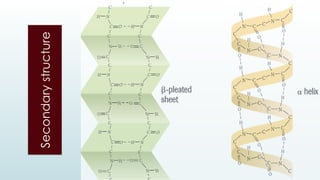
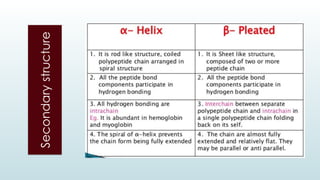
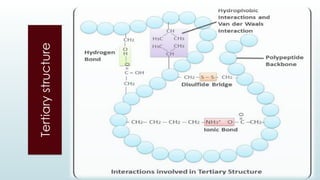
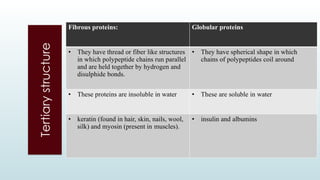
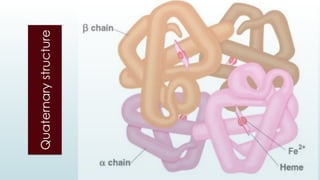
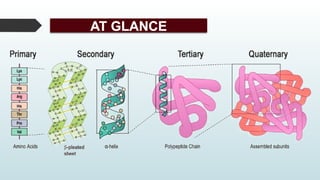
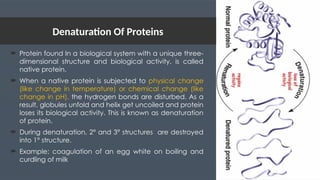
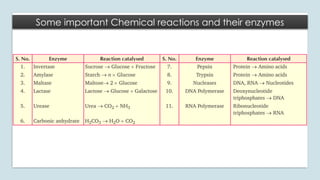
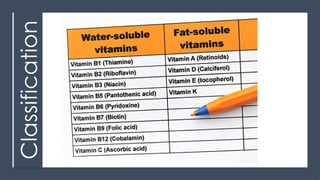
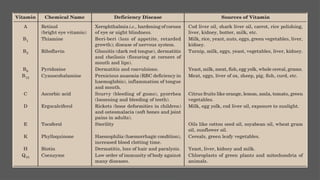
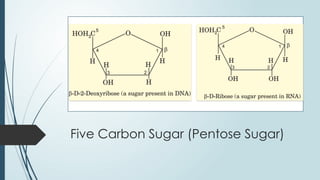
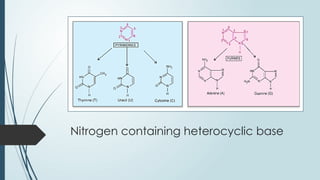
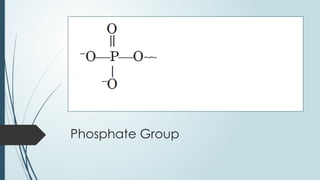
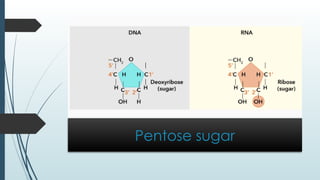
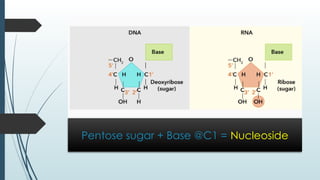
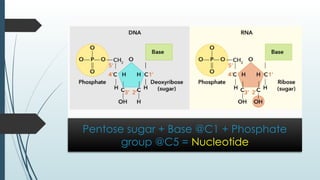
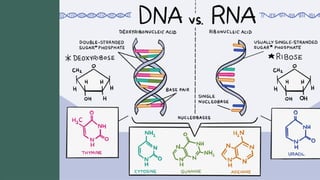
The document discusses biomolecules, focusing on carbohydrates, proteins, vitamins, and nucleic acids. It classifies carbohydrates into monosaccharides, oligosaccharides, and polysaccharides, detailing their structures, hydrolysis, and examples. Additionally, it explains proteins' structures and functions, the role of enzymes, and the significance of vitamins in biological processes.