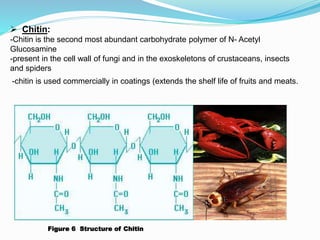
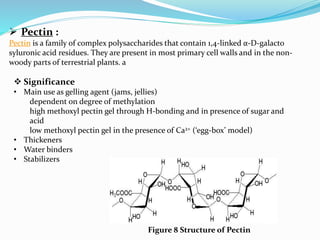
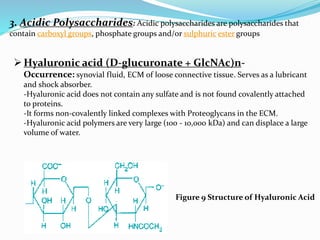
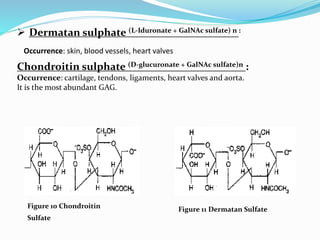
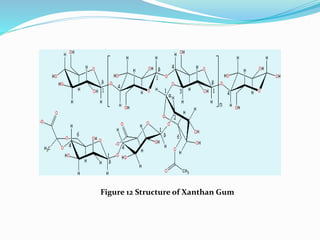
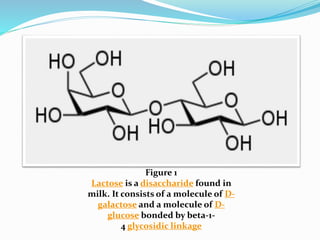
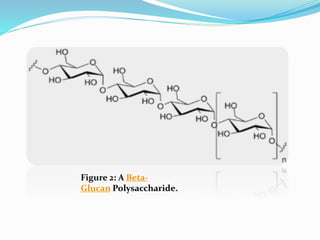
This document provides information about polysaccharides. It defines polysaccharides as natural condensation polymers composed of long chains of monosaccharides. Polysaccharides are classified as homopolysaccharides or heteropolysaccharides depending on whether they are composed of one type of monosaccharide or multiple types. Examples of important polysaccharides are discussed, including starch, glycogen, cellulose, chitin, pectin, and hyaluronic acid. Their structures and functions in storage, structure, and as acidic polymers are described in 1-3 sentences for each.





![1. Storage
Polysaccharides:
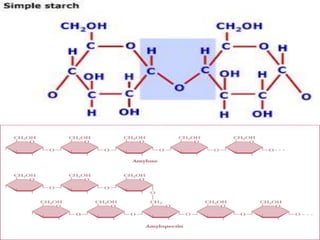
Starch [(C6H10O5)n] : -
Most common storage polysaccharide in plants
-Composed of 10 – 30% Amylose and 70-90% Amylopectin depending on the source
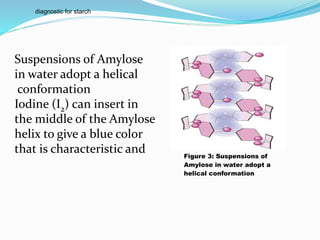
(a) Amylose is a linear polymer of α-D-glucose, linked together by α 1→4 glycosidic
linkages. It is soluble in water, reacts with iodine to give a blue color and the
molecular weight of Amylose ranges between 50, 000 – 200, 000.
(b) Amylopectin is a highly branched polymer, insoluble in water, reacts with iodine
to give a reddish violet color. The molecular weight ranges between 70, 000 - 1 000,
000. Branches are composed of 25-30 glucose units linked by α 1→4 glycosidic linkage
in the chain and by α 1→6 glycosidic linkage at the branch point.](https://image.slidesharecdn.com/presentation1-161013073758/85/Presentation-on-polysaccharides-9-320.jpg)

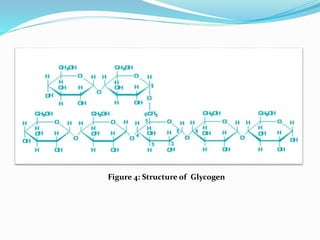
![ Glycogen [C24H42O21]:
-Also known as animal starch
-Stored in muscle and liver
-Present in cells as granules (high MW)
-Contains both α(1,4) links and α (1,6) branches at every 8 to 12 glucose unit
-Complete hydrolysis yields glucose
- With iodine gives a red-violet color
-Hydrolyzed by both α and β-amylases and by glycogen phosphorylase
Significance-
-In the liver, glycogen synthesis and degradation are regulated to maintain
blood-glucose levels as required to meet the needs of the organism as a whole.
Glycogen serves as a buffer to maintain blood glucose level.
-In contrast, in muscle, these processes are regulated to meet the energy needs
of the muscle itself.
- The concentration of glycogen is higher in the liver than in muscle (10% versus
2% by weight), but more glycogen is stored in skeletal muscle overall because of
its much greater mass.](https://image.slidesharecdn.com/presentation1-161013073758/85/Presentation-on-polysaccharides-13-320.jpg)

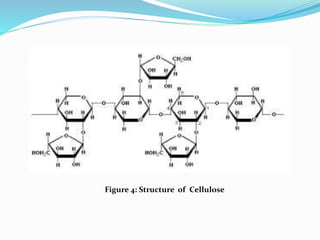
![2. Structural Polysaccharides:
Cellulose [(C6H10O5)n]:
-Polymer of b-D-glucose linked by b(1,4) linkages
-Yields glucose upon complete hydrolysis
-Partial hydrolysis yields cellobiose
-Most abundant of all carbohydrates
-Gives no color with iodine
-Cellulose is tasteless, odorless and insoluble in water and
most organic solvents.
Significance—
Microcrystalline cellulose: used as binder- disintegrant in tablets
-Methyl cellulose: suspending agent and bulk laxative
-Oxidized cellulose: hemostat
-Sodium carboxymethyl cellulose: laxative
-Cellulose acetate: rayon; photographic film; plastics
-Cellulose acetate phthalate: enteric coating
-Nitro cellulose: explosives; collodion (pyroxylin)](https://image.slidesharecdn.com/presentation1-161013073758/85/Presentation-on-polysaccharides-16-320.jpg)