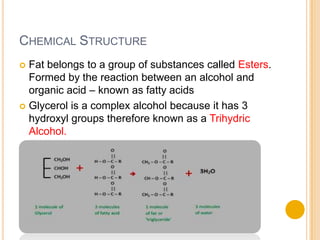
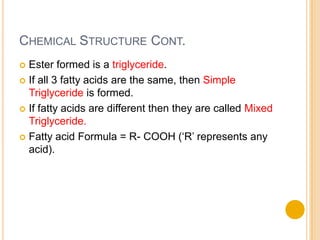
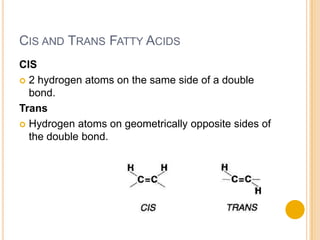
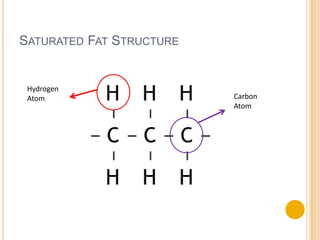
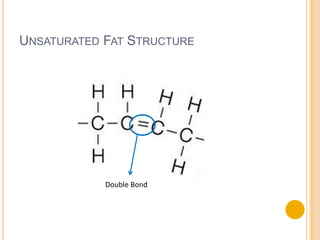
Lipids are made up of carbon, hydrogen and oxygen and serve several functions in the body including as an energy source. They are found in foods like meat, fish, dairy and processed foods. Chemically, lipids are triglycerides composed of a glycerol molecule bonded to three fatty acid molecules. Fats can be saturated, monounsaturated or polyunsaturated depending on the number of double bonds in the fatty acids. Essential fatty acids must be obtained through diet and are important for cell membrane structure and hormone production.