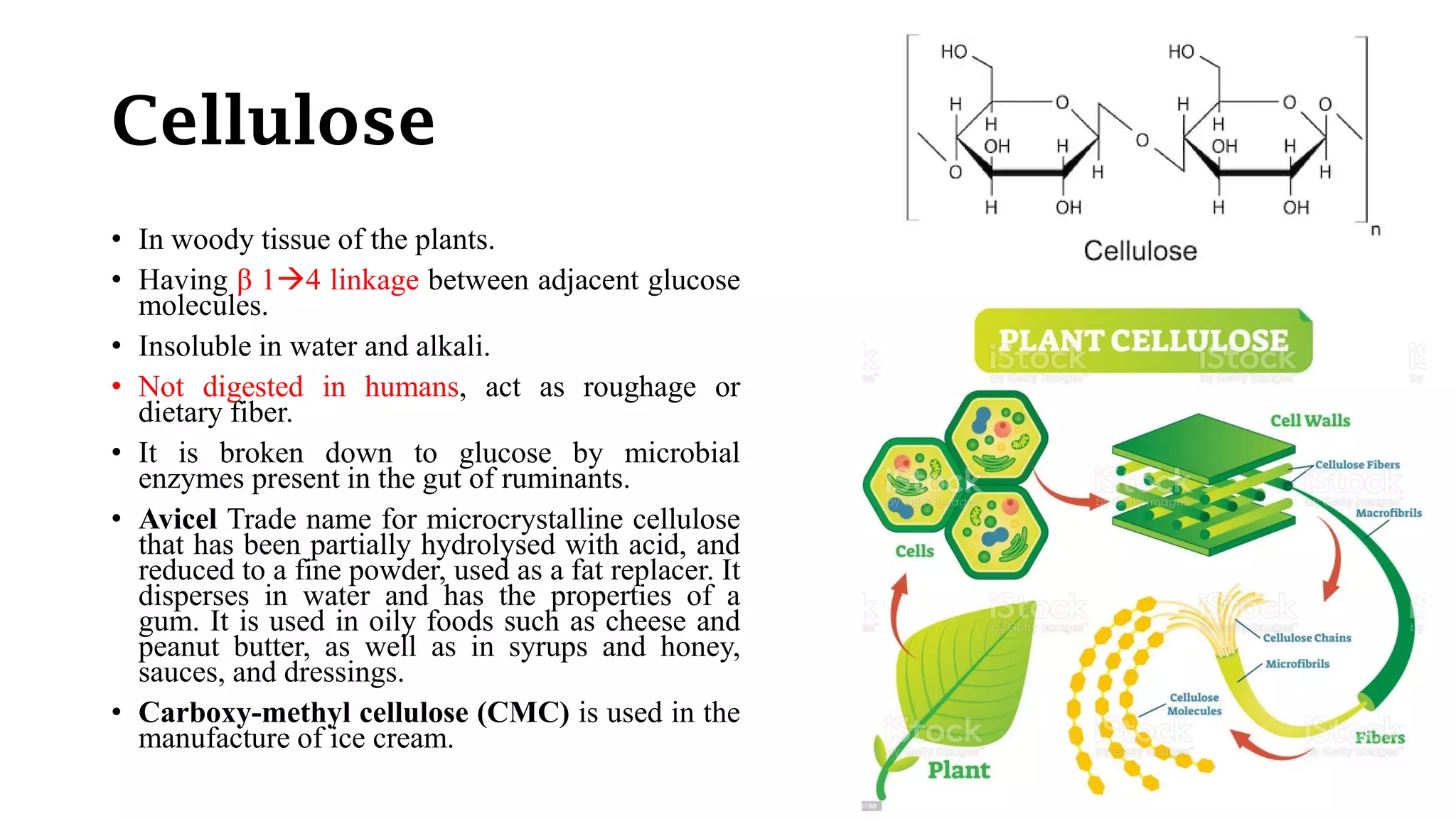
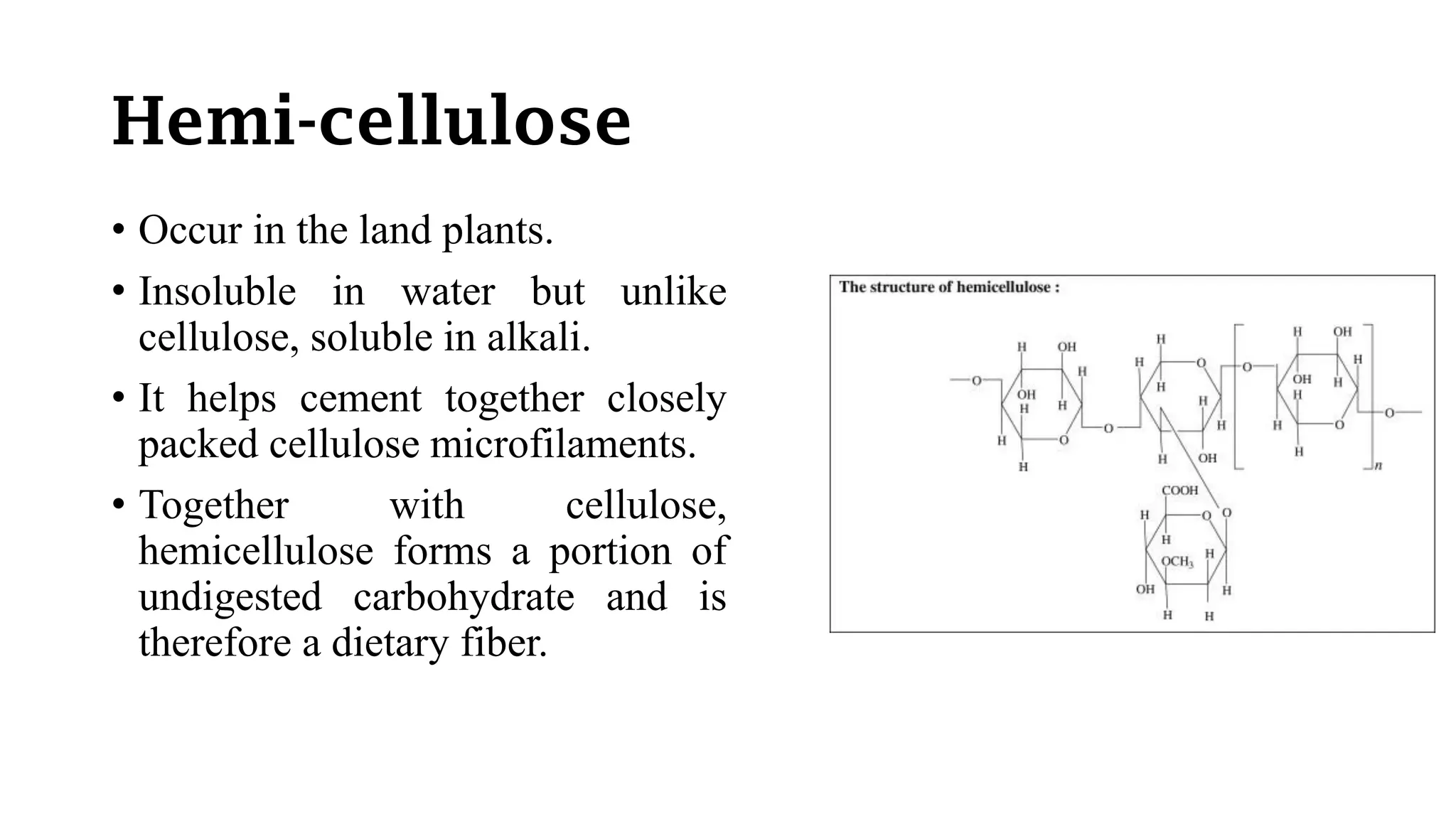
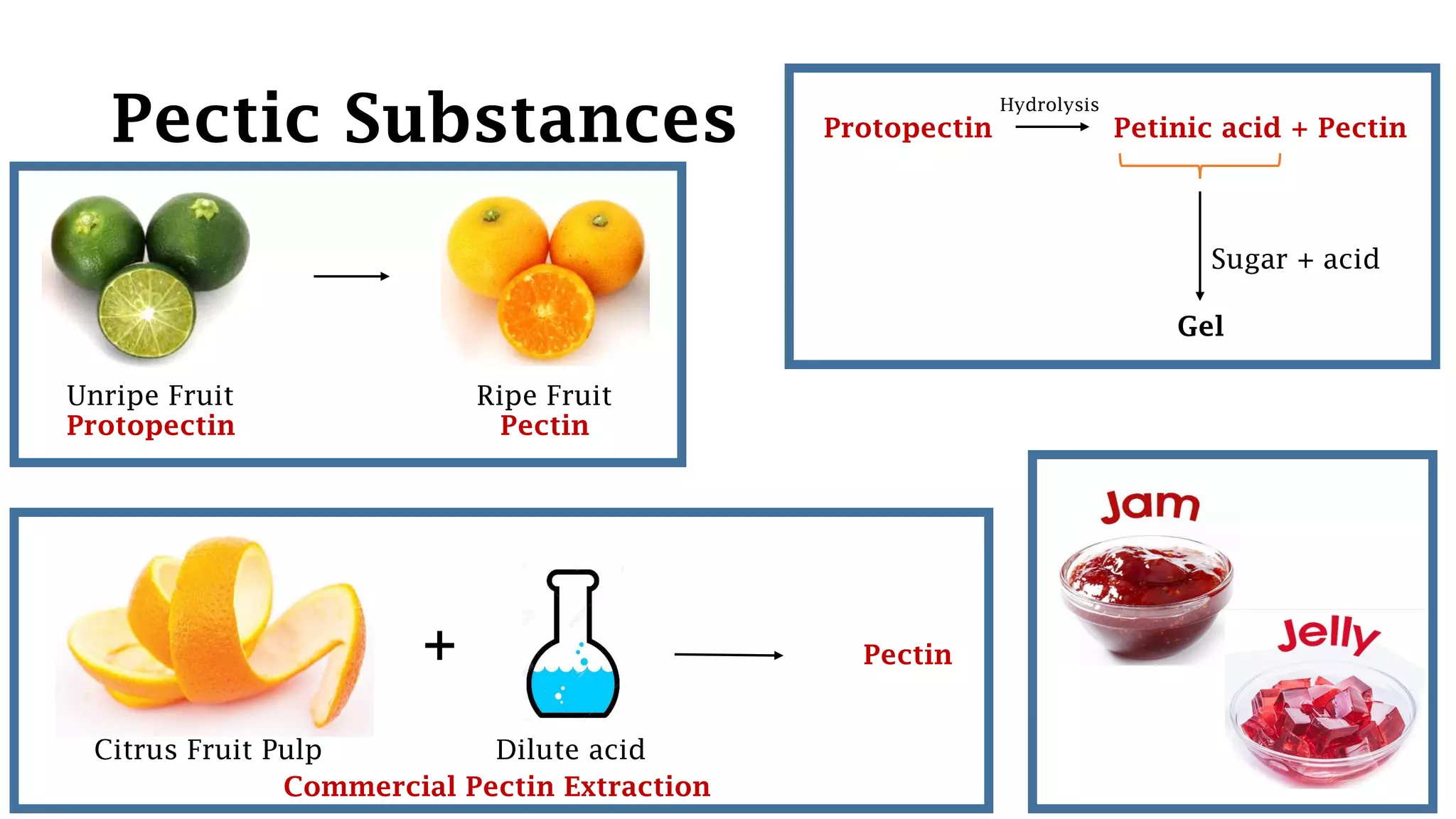
Polysaccharides are high molecular weight carbohydrates composed of numerous monosaccharide units, commonly found as starch, glycogen, and cellulose among others. They play crucial roles in food as storage substances, thickeners, and dietary fibers, impacting texture, digestibility, and water retention. Specific polysaccharides, such as amylose and amylopectin, influence various properties like gelatinization and retrogradation, affecting food quality and shelf-life.