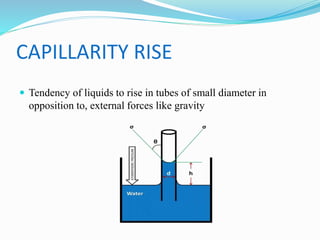
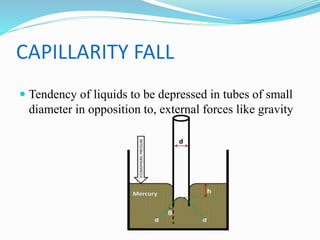
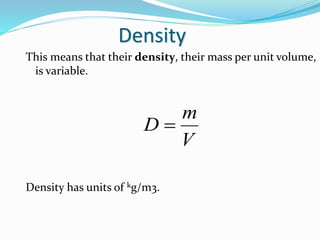
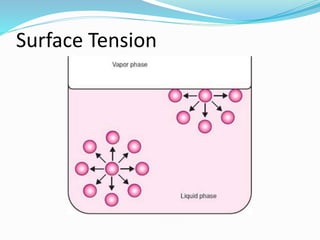
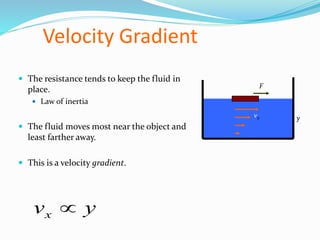
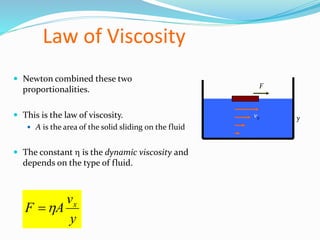
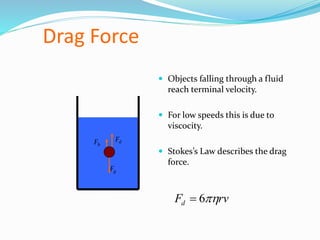
This document discusses several fluid properties including capillarity, density, surface tension, and viscosity. It defines capillarity as the ability of a liquid to flow in narrow spaces against forces like gravity. Capillarity can occur through rise or fall and is responsible for phenomena like water moving up plants and oil rising in wicks. Surface tension is caused by stronger attractive forces between liquid molecules than between liquids and gases, creating inward pressure on the surface. Viscosity is a fluid's resistance to flow, defined by Newton's law of viscosity as proportional to the velocity gradient. It describes the drag force on objects moving through fluids.