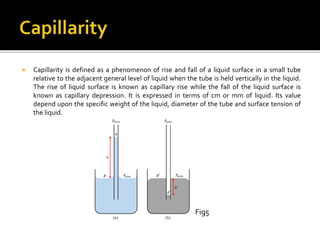
The document discusses surface tension, which is the tensile force acting on a liquid's surface that causes it to behave like a membrane. It outlines the historical contributions of Agnes Pockels to the field, explains phenomena such as capillary rise and fall in small tubes, and describes the balance of forces around liquid droplets and jets. Illustrative figures and equations are included to support the explanations of these concepts.