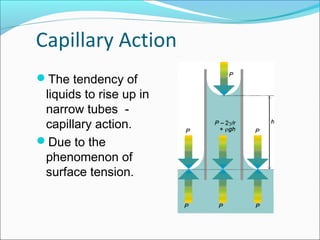
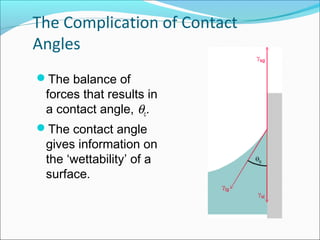
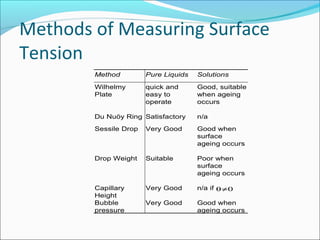
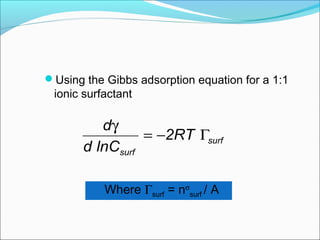
This document discusses surface tension and methods for measuring surface tension. It defines surface tension as the imbalance of intermolecular forces at a liquid-air interface. Several common methods for measuring surface tension are described, including the Wilhelmy plate method, Du Nuoy ring method, sessile drop method, and maximum bubble pressure method. Factors that influence surface tension such as temperature and presence of surfactants are also covered.