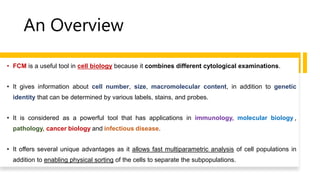
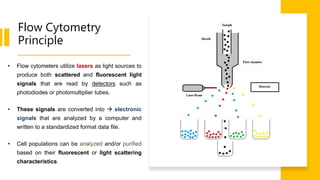
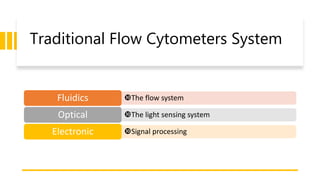
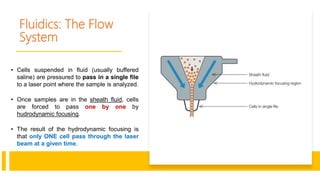
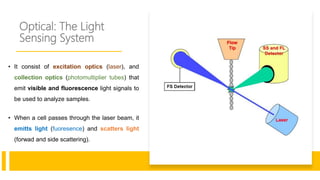
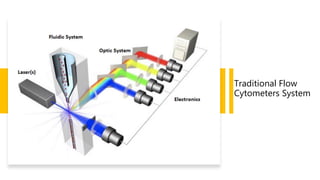
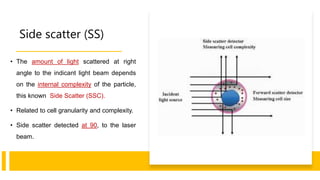
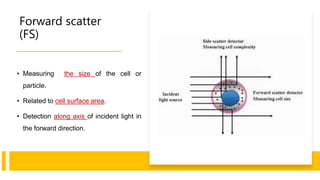
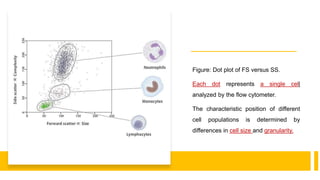
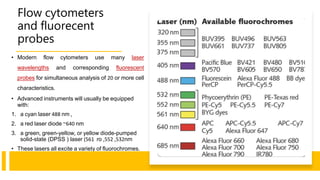
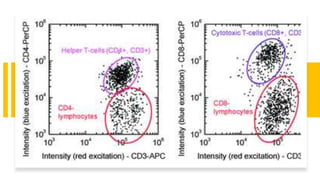
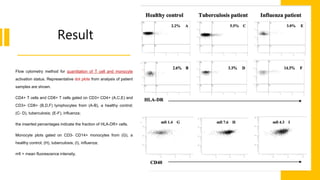
Flow cytometry is a technique used in cell biology that allows for the analysis of physical and chemical characteristics of cells as they flow in a fluid stream through a beam of light. It provides rapid multi-parametric analysis of cells based on light scattering, fluorescence, and other optical properties. Flow cytometry gives information about cell size, granularity, and the expression of cell surface markers or intracellular proteins through the use of fluorescent probes. It has many applications in fields like immunology, cancer research, and infectious disease.