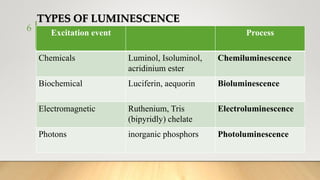
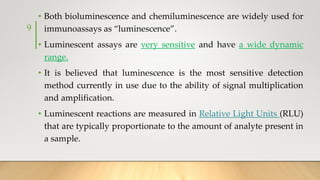
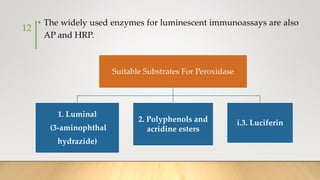
The document discusses the principles and applications of chemiluminescence, focusing on its use in immunoassays and various biochemical analyses. It outlines the mechanisms of chemiluminescence, enhanced chemiluminescence, and electrochemiluminescence, as well as the types of substrates and enzymes employed. Additionally, the document addresses the benefits, limitations, and specific applications of chemiluminescence in fields such as forensic science and clinical diagnostics.





![Emission of light with limited emission of heat (luminescence), as the result of
a chemical reaction.
[A] + [b] → [◊] → [products] + light
[A], [b]: reactants [◊]: Excited intermediate
For example, if [A] is luminol and [B] is hydrogen peroxide in the presence of
a suitable catalyst we have:
Luminol + H2O2 →3-APA[◊] →3-APA + light
Where:
3-APA is 3-aminophthalate
3-APA[◊] is the excited state producing light as it decays to a lower energy
level.
8](https://image.slidesharecdn.com/chemiluminescence-171010065425/85/Chemiluminescence-8-320.jpg)

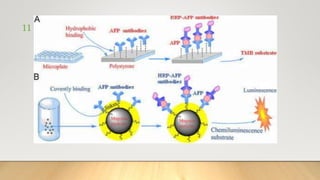

![• Luminol oxidation reaction is carried out in an alkaline buffer,
peroxidase enzymes and reactive oxygen species [peroxide anion
(O2-), singlet oxygen (1O2), hydroxyalkyl radical (OH •), peroxide
hydrogen (H2O2)], to generate excited state intermediates.
• When they return to the ground state, a wavelength of 425 nm is
emitted. Typically, light emission stabilizes in less than 2 minutes,
and sustained emission lasts for approximately 20 minutes or
more.
14](https://image.slidesharecdn.com/chemiluminescence-171010065425/85/Chemiluminescence-14-320.jpg)





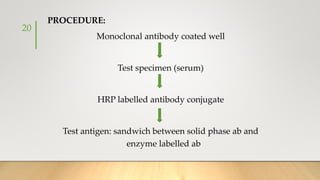





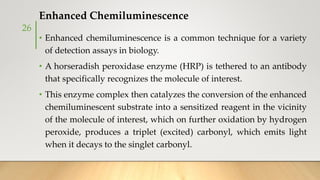



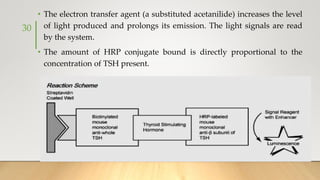
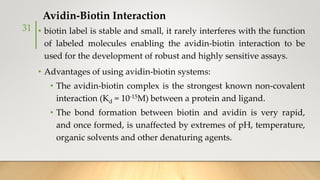
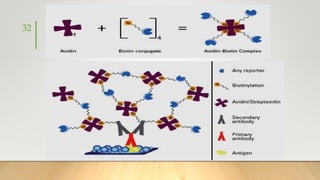



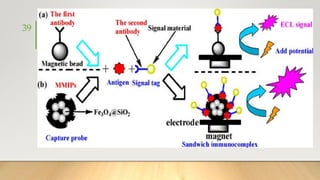
![• It generally uses Ruthenium complexes, especially [Ru (Bpy)3]
regenerating with TPrA (Tripropylamine) in liquid phase or liquid–
solid interface.
• It can be used as monolayer immobilized on an electrode surface or
as a coreactant or more commonly as a tag and used in HPLC,
Ru tagged antibody based immunoassays,
Ru Tagged DNA probes for PCR,
NADH or H2O2 generation based biosensors,
oxalate and organic amine detection
and many other applications and can be detected from
picomolar sensitivity to dynamic range
37](https://image.slidesharecdn.com/chemiluminescence-171010065425/85/Chemiluminescence-37-320.jpg)