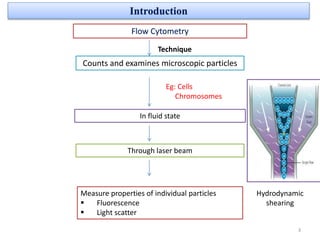
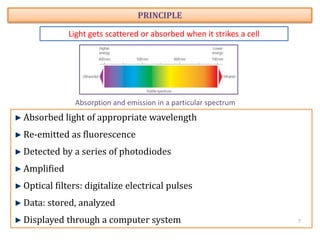
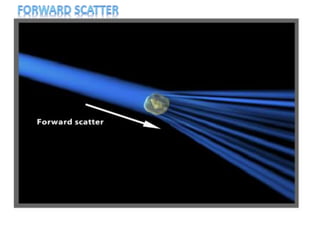
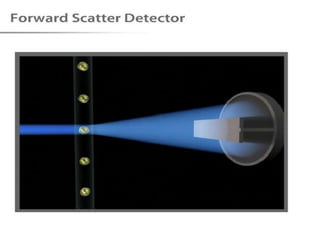
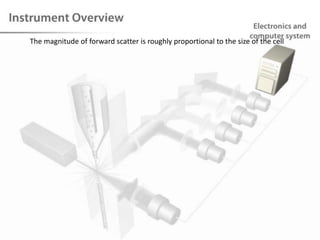
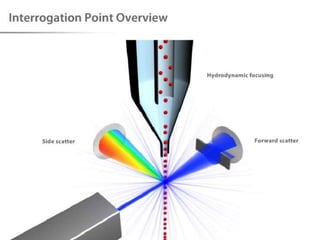
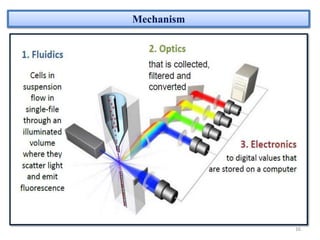
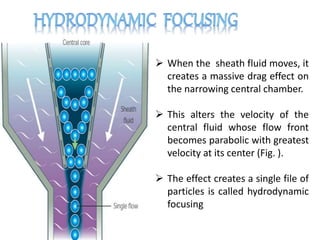
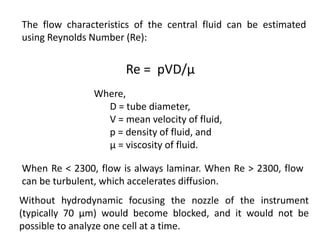
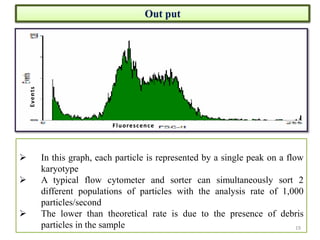
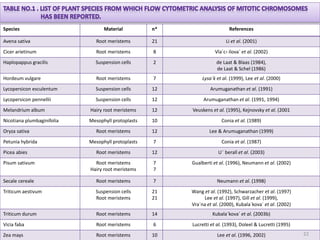
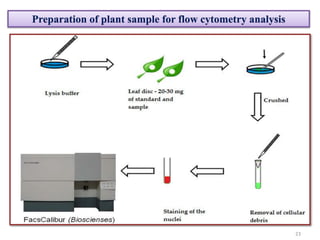
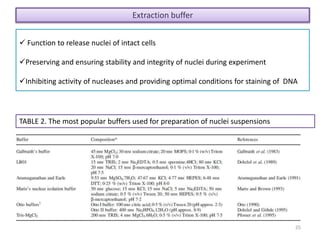
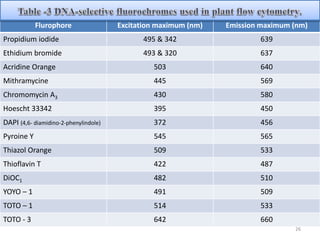
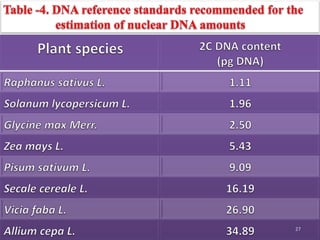
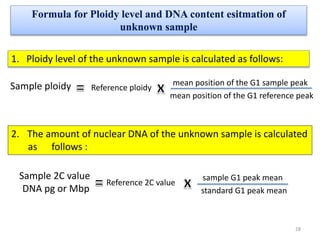
The document discusses flow cytometry, a technique for analyzing microscopic particles such as cells in a fluidic state, detailing its historical development, principles, and operational mechanics. It describes how flow cytometers can analyze multiple parameters per cell at high speeds and includes information on sample preparation, quality factors, and various applications in plant research. Key concepts like fluorescence, hydrodynamic focusing, and DNA content estimation are also covered.