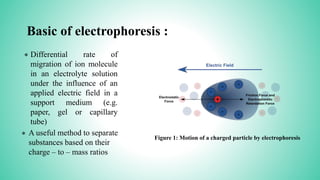
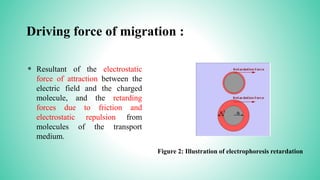
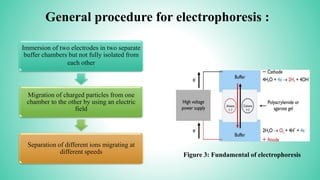
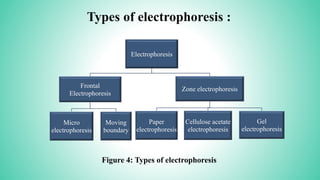
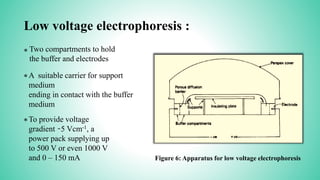
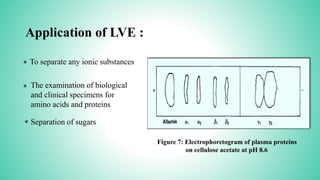
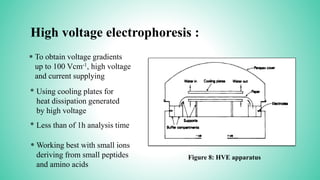
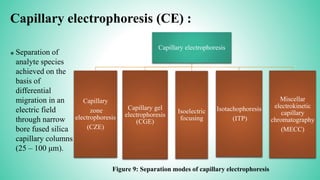
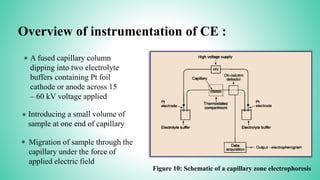
This presentation provides an overview of electrophoresis. It begins by defining electrophoresis as the differential migration of ionized molecules in an electric field based on their charge-to-mass ratio. It then discusses the basic principles, factors affecting mobility, supporting media like paper and gels, common techniques including low voltage and capillary electrophoresis, and applications for analyzing proteins, DNA, antibiotics and more. The presentation concludes that electrophoresis is a useful method for separating charged substances, from small ions to large molecules, and is widely used despite some limitations.