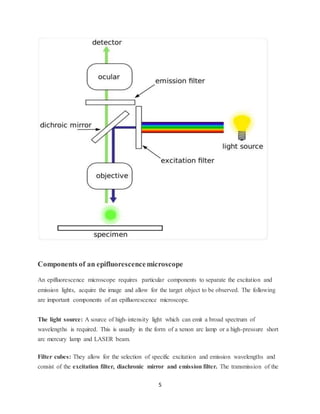
Fluorescence microscopy uses fluorescent dyes and high intensity light to illuminate samples and produce magnified images based on the emitted light from fluorophores rather than the illuminating light. There are several types of fluorescence microscopy including epifluorescence microscopy, confocal microscopy, two-photon excitation microscopy, and total internal reflection fluorescence microscopy. Epifluorescence microscopy is the most basic type and uses filter cubes to separate excitation and emission wavelengths. Confocal microscopy increases resolution by using a pinhole to reject out-of-focus light. Two-photon excitation microscopy penetrates deeper by using two lower energy photons simultaneously for excitation rather than one high energy photon. Total internal reflection fluorescence microscopy images fluorophores very close to a glass