The document is the notes from an atomic spectrum class that include the agenda, objectives, and content about atomic spectra. The key points are:
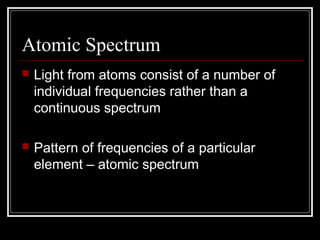
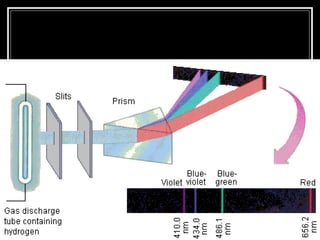
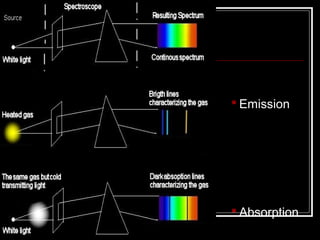
1. Atoms emit and absorb specific frequencies of light that make up their atomic spectrum, which acts as a fingerprint to identify elements.
2. Flame tests can identify elements based on the color of light they emit.
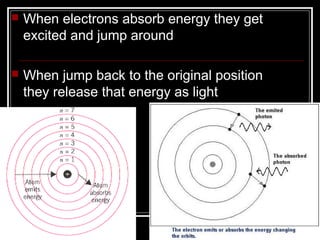
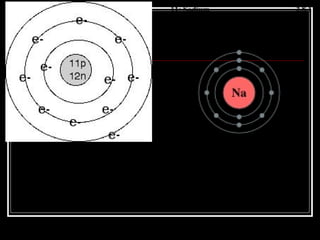
3. The Bohr model describes electrons absorbing and emitting energy in quantized levels as they move between orbits, releasing photons of specific frequencies.