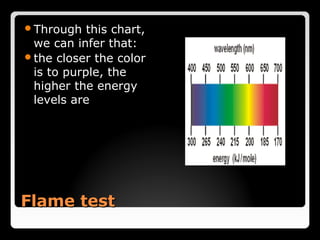
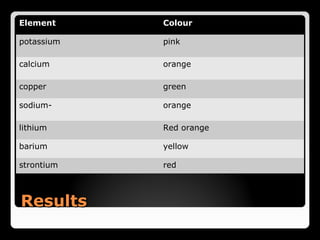
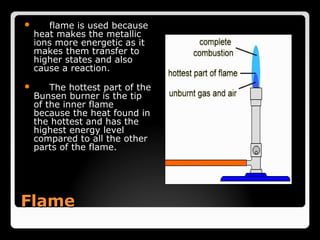
This document describes the procedure and observations of a flame test experiment. The experiment involves placing various metal salt solutions on a nichrome wire and exposing the wire to a flame to observe the color of the flame. Each metal ion produces a unique color that can be used to identify the metal. Some difficulties noted are that some colors are similar, and samples with multiple metals produce mixed colors that are difficult to interpret. Real-world applications of flame tests discussed are in fireworks to produce various colored explosions, and in forensic science to identify metals in blood samples.