Embed presentation
Downloaded 36 times


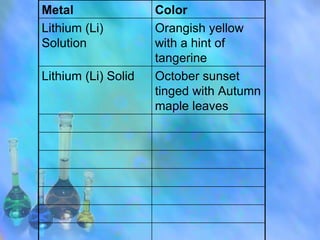


Different elements emit unique atomic line spectra colors when burned, due to electrons gaining and losing discrete energy levels and releasing photons of specific frequencies. Lithium burns with an orangish-yellow hue, demonstrating flame tests can distinguish elements based on their colored flames. Questions ask why chemicals have different colored flames, why heating is needed before light emission, and how metals create the color in salts.




