The document details the history and development of atomic models from ancient concepts by Democritus to modern quantum mechanical theories, highlighting key figures like Dalton, Thomson, Rutherford, Bohr, Sommerfeld, Schrödinger, and Chadwick. It discusses the structure of atoms, including subatomic particles (protons, neutrons, and electrons), their properties, and the principles guiding electron behavior and chemical bonding. Additional mentions include various theories related to electron configuration, atomic mass, and types of chemical bonds.

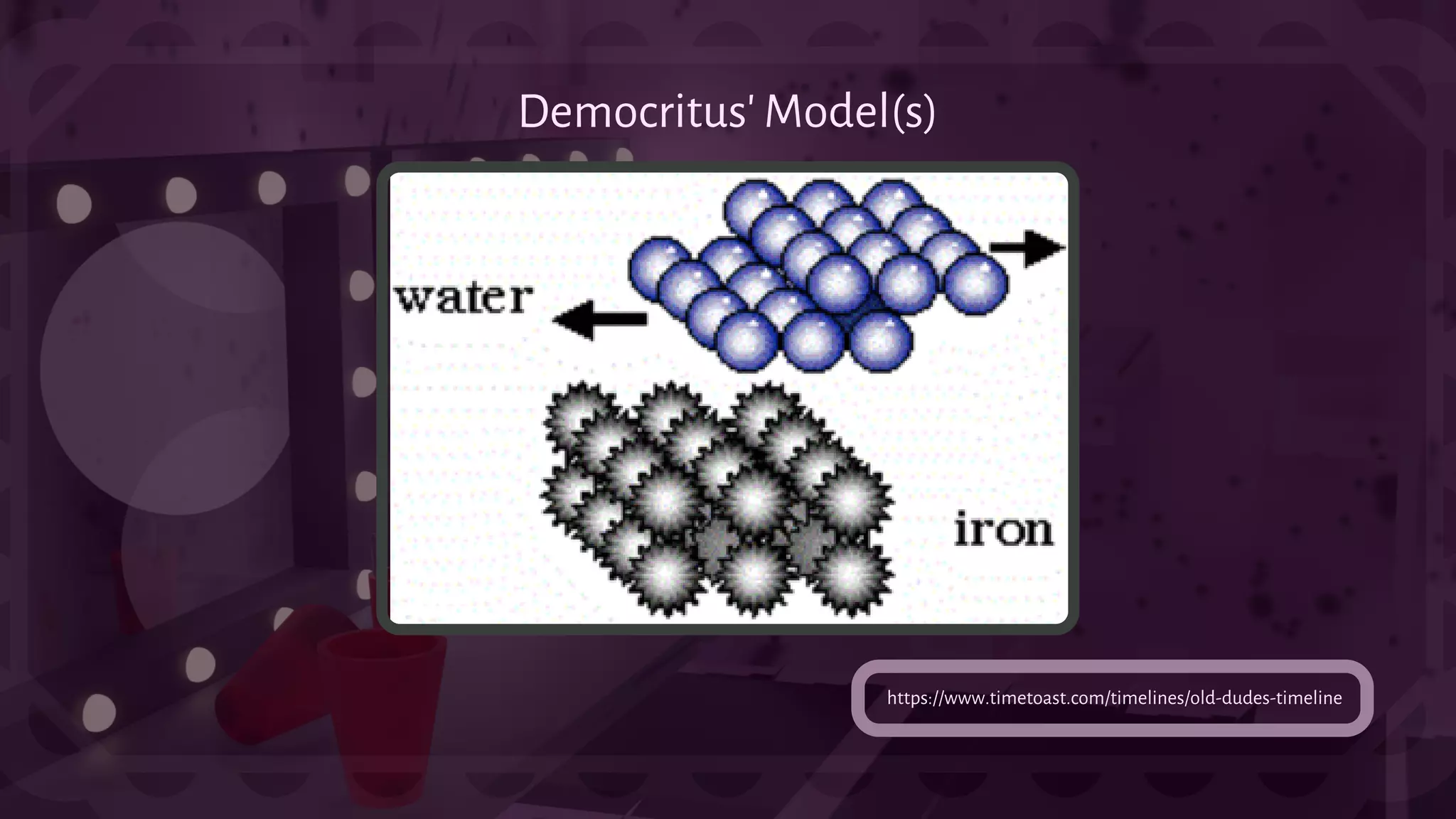
![- An Atom is a fundamental thing in matter as
it serves so much purpose from being the basic
unit and defining structure of an element to
making up everything in the universe. This is
also the source of nuclear energy.
Atom:[ˈadəm] Greek - Indivisible, Uncuttable
({atomos} )](https://image.slidesharecdn.com/science-4science2ndquarter-220308080142/75/Science-Grade-9-2nd-Quarter-3-2048.jpg)

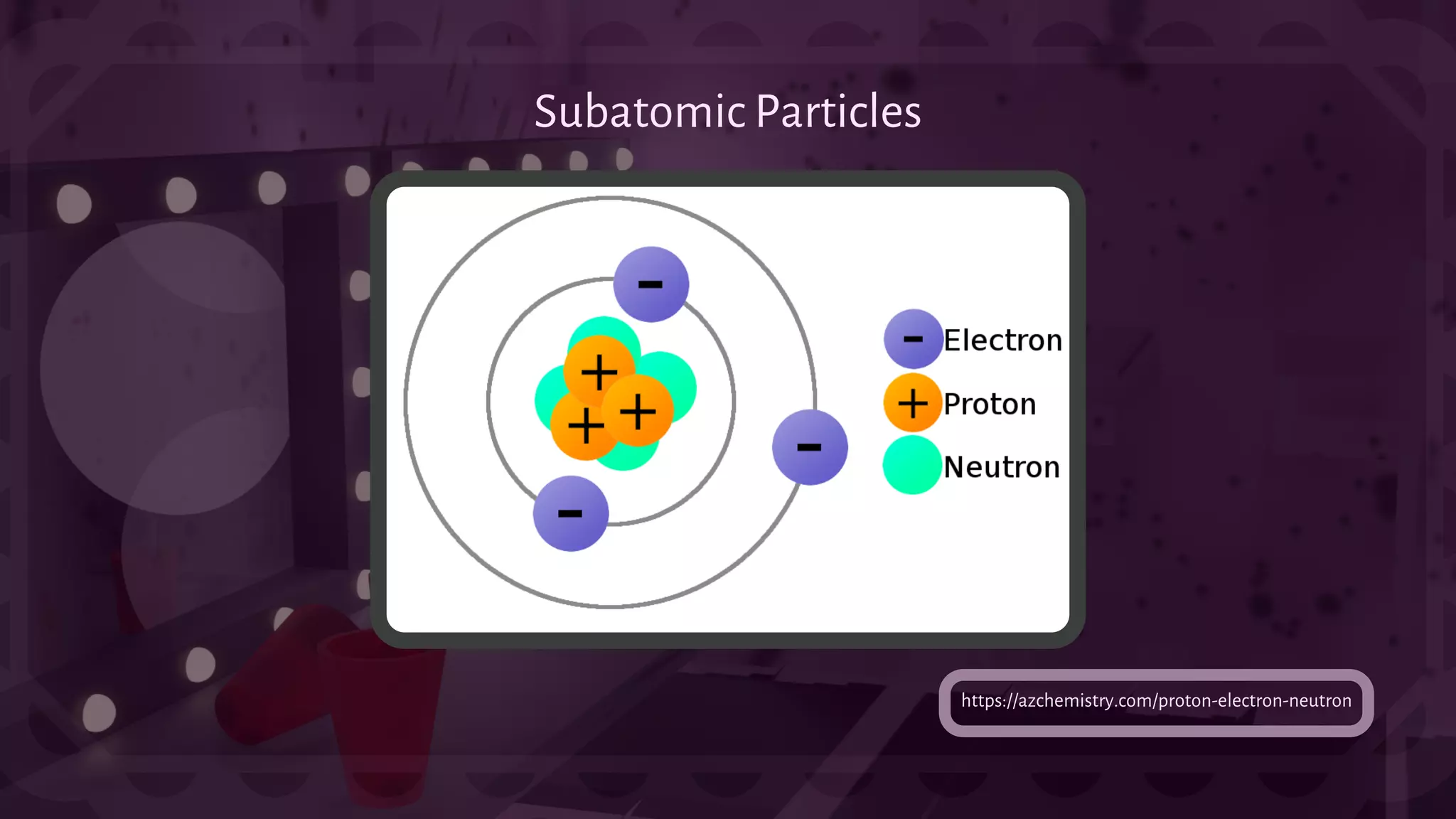
![- An Atom is made up of subatomic particles, more specifically called Proton [prō
ˌtän], Neutron [n(y)o͞ oträn], and Electron [əˈlekˌträn]. (May not be accurate.) The
Greek words and meaning for each particles (If there are any.) respectively are:
({prôtos} First), ({elektron?} Amber).
- In the center of the Atom, protons and neutrons reside inside a part of an Atom
called a nucleus. The electrons however, are located over the nucleus, flying
around in a tiny cloud. Protons have a positive charge, neutrons have no net
charge, and electrons have a negative charge. A neutral atom consists of equal
amounts of protons and electrons, these also often consist of an equal amount of
neutrons.
Subatomic Particles:](https://image.slidesharecdn.com/science-4science2ndquarter-220308080142/75/Science-Grade-9-2nd-Quarter-5-2048.jpg)

























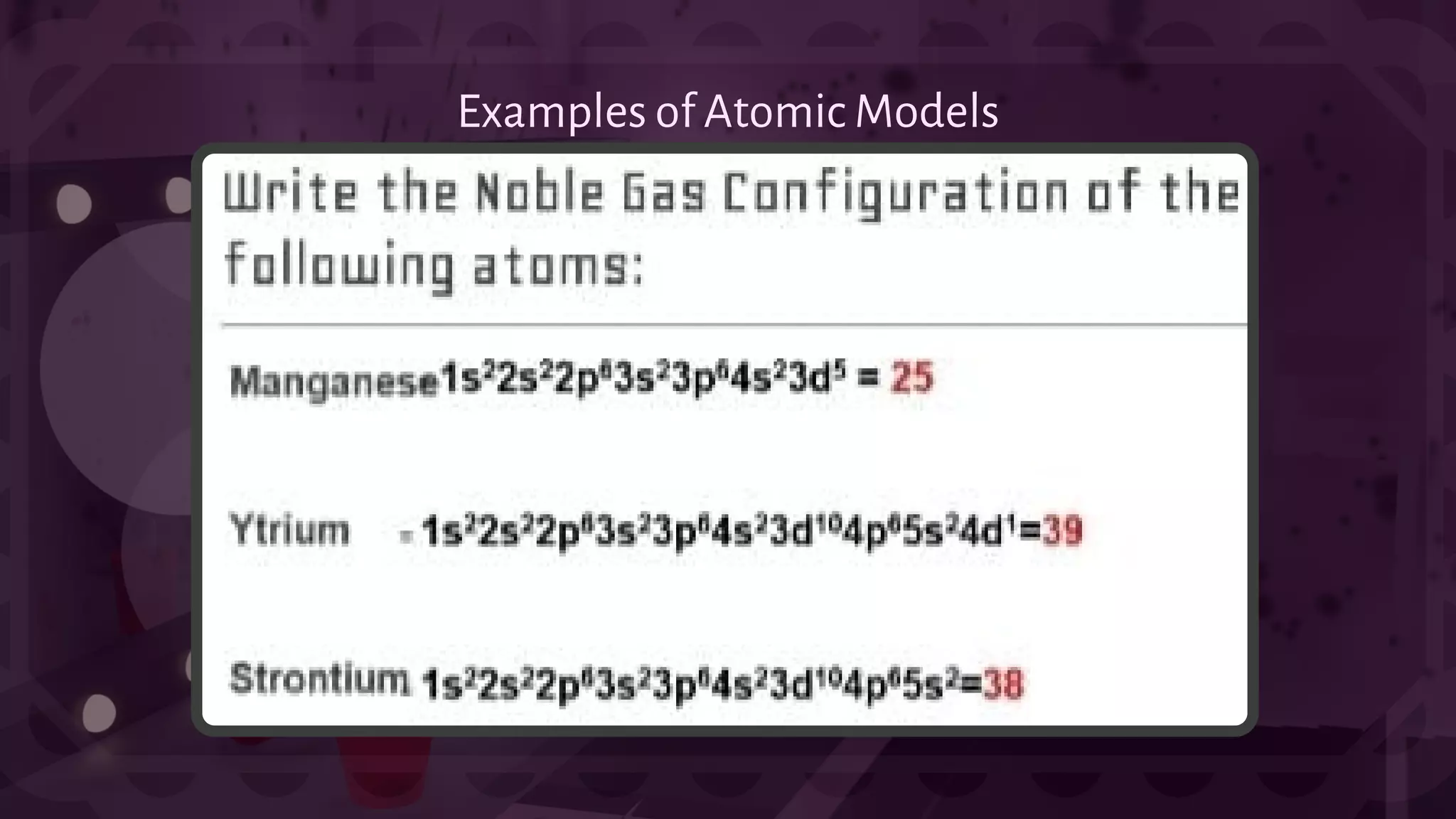




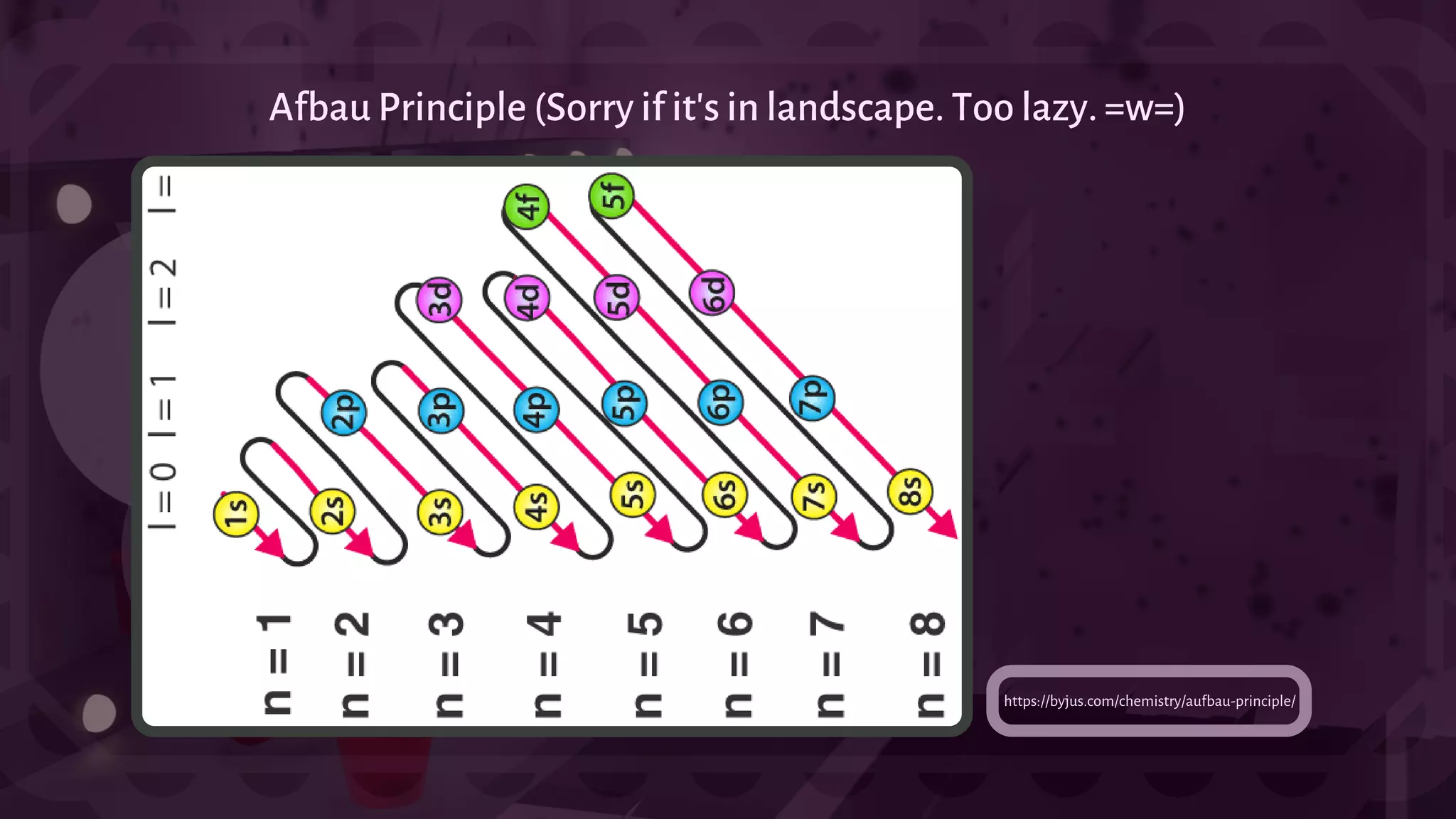

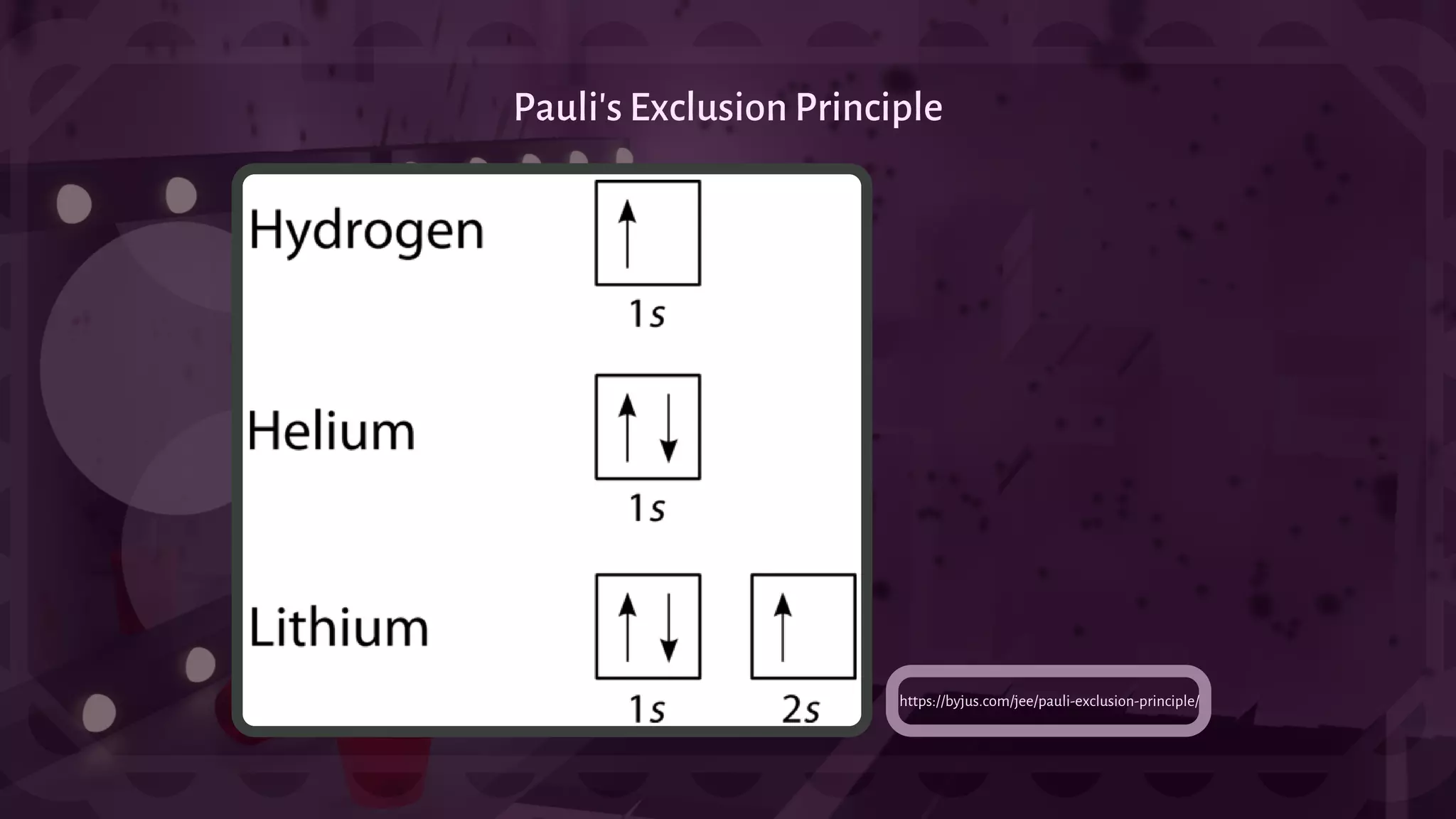

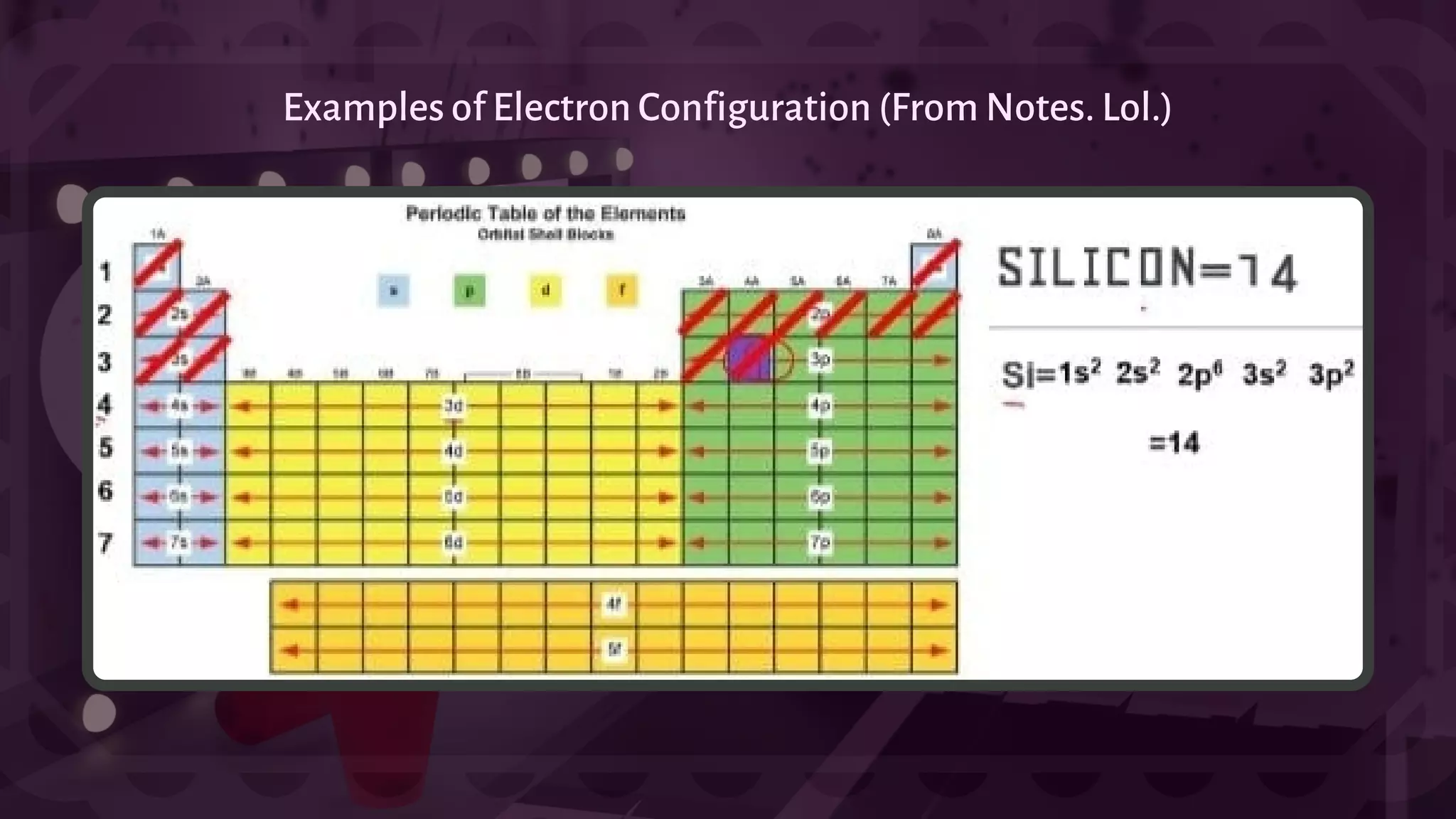
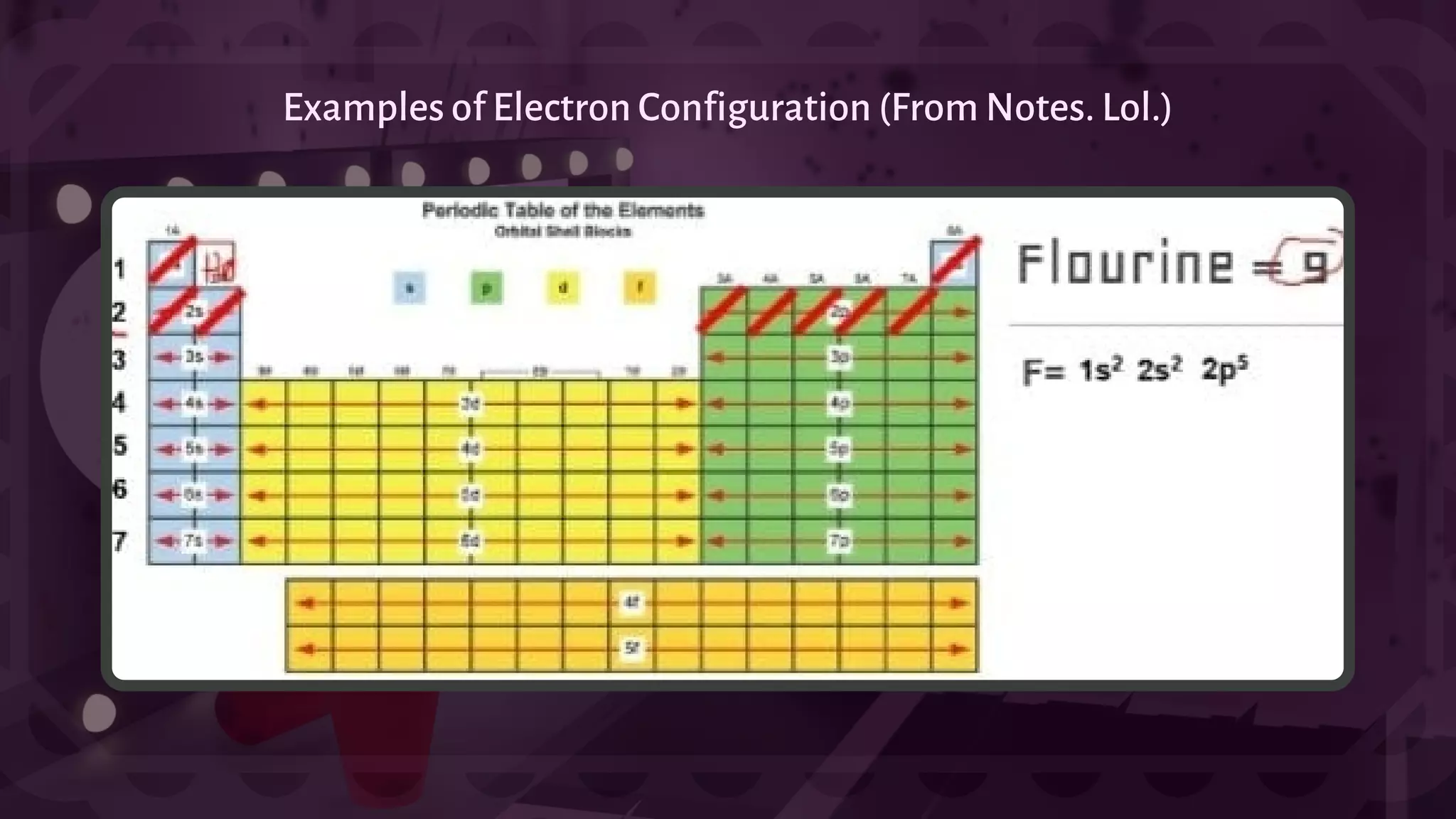

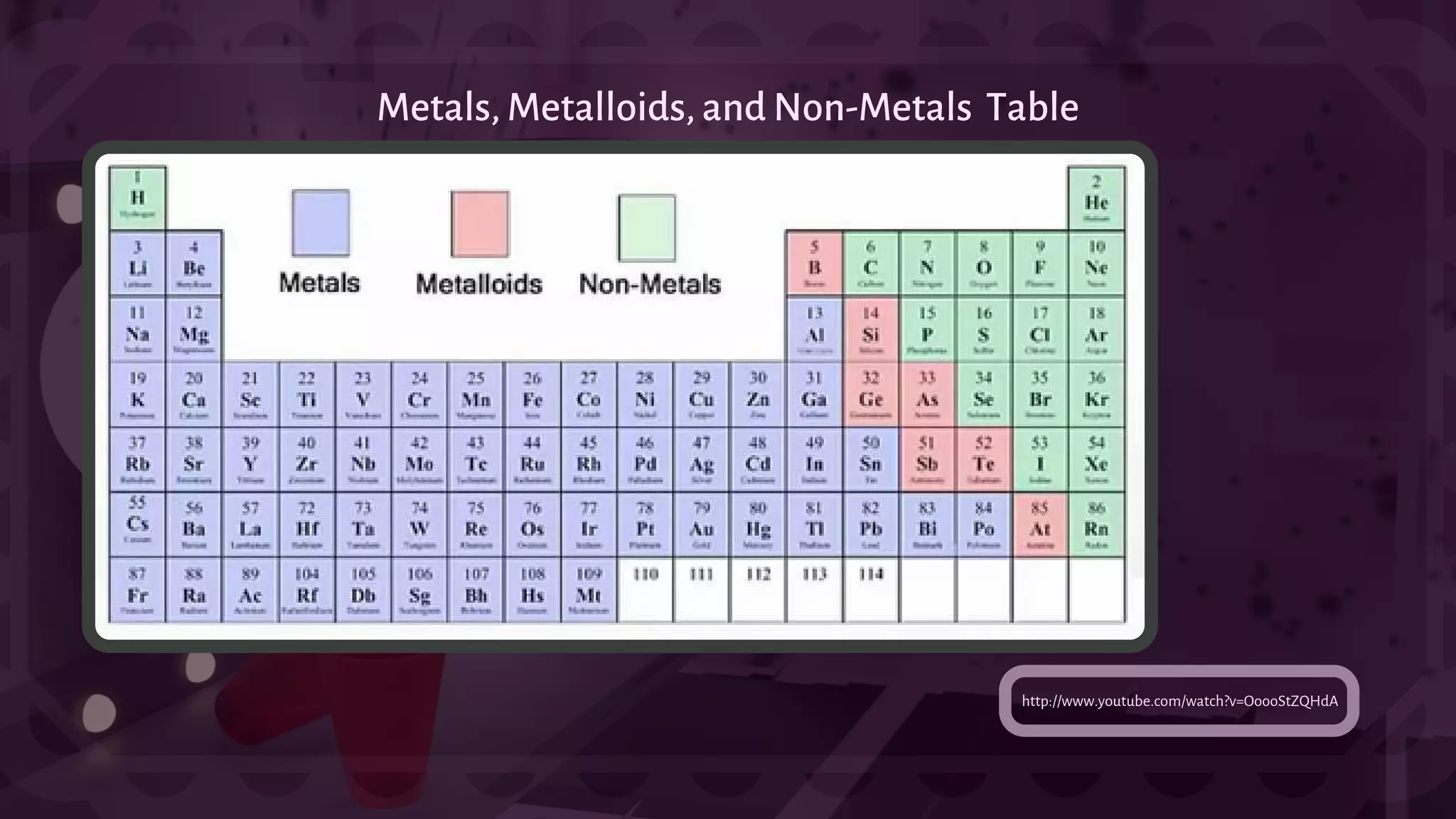


![- A type of chemical bonding called Ionic Bonding that involves the electrostatic attraction between
oppositely charged ions, or between two atoms with sharply different electronegativities,[1] and is the primary
interaction occurring in ionic compounds.
- Metallic bond is a term used to describe the collective sharing of a sea of valence electrons between several
positively charged metal ions.
- Covalent Bonding is formed by equal sharing of electrons from both the participating atoms. The pair of
electrons participating in this type of bonding is called shared pair or bonding pair. The covalent bonds are also
termed as molecular bonds. Sharing of bonding pairs will ensure that the atoms achieve stability in their outer
shell which is similar to the atoms of noble gases.
Three Major Types of Chemical Bonding](https://image.slidesharecdn.com/science-4science2ndquarter-220308080142/75/Science-Grade-9-2nd-Quarter-52-2048.jpg)