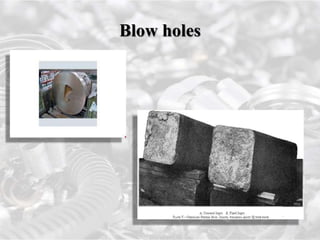
This document discusses ferrous metals, which include iron, steel, and their alloys. It describes the production of pig iron through the blast furnace process and its uses. Pig iron can be further processed into cast iron and wrought iron. Steel is also discussed, which contains 0.1-1.5% carbon and is produced through various processes including the Bessemer process. The properties and applications of these ferrous metals are explained.