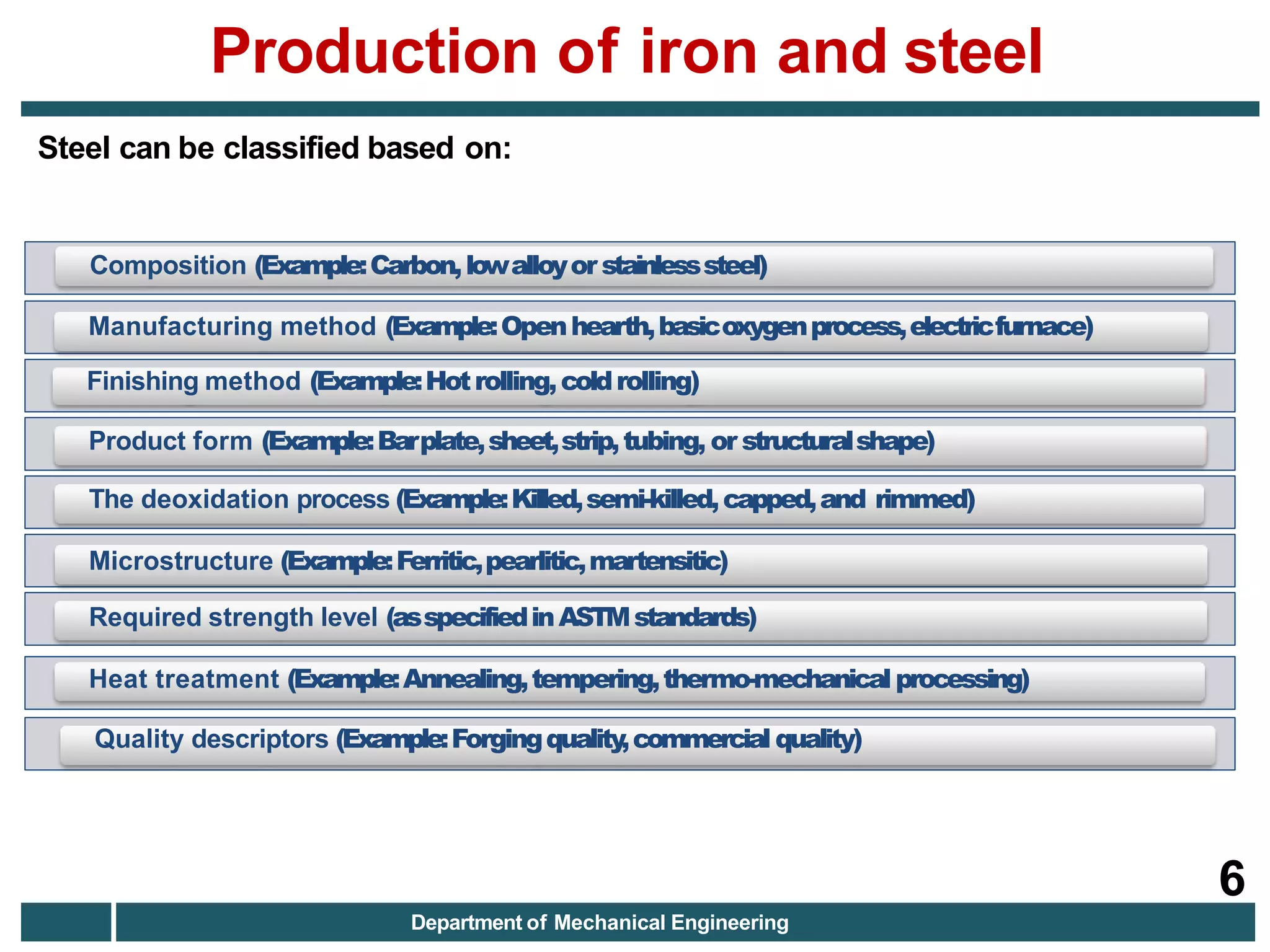
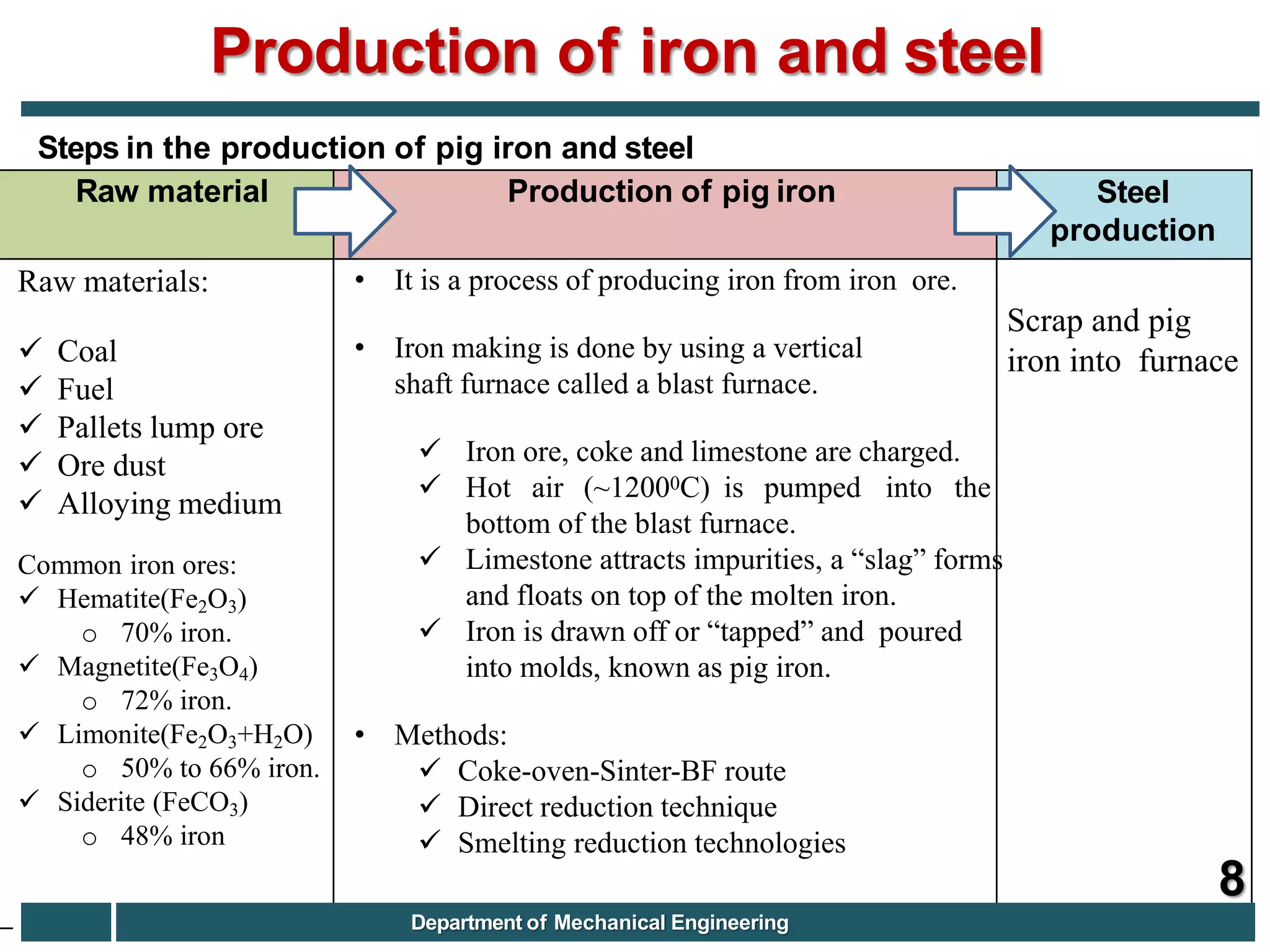
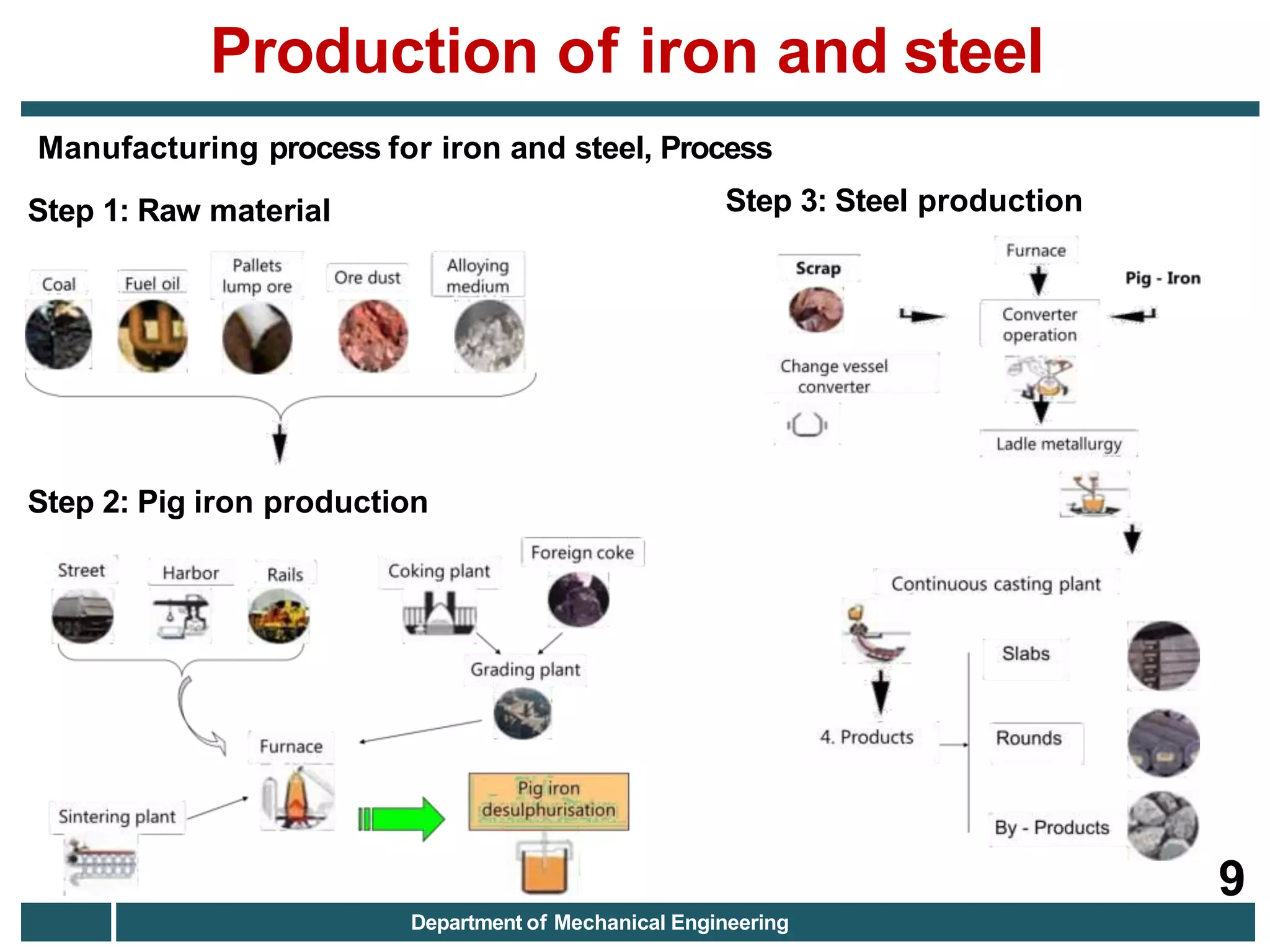
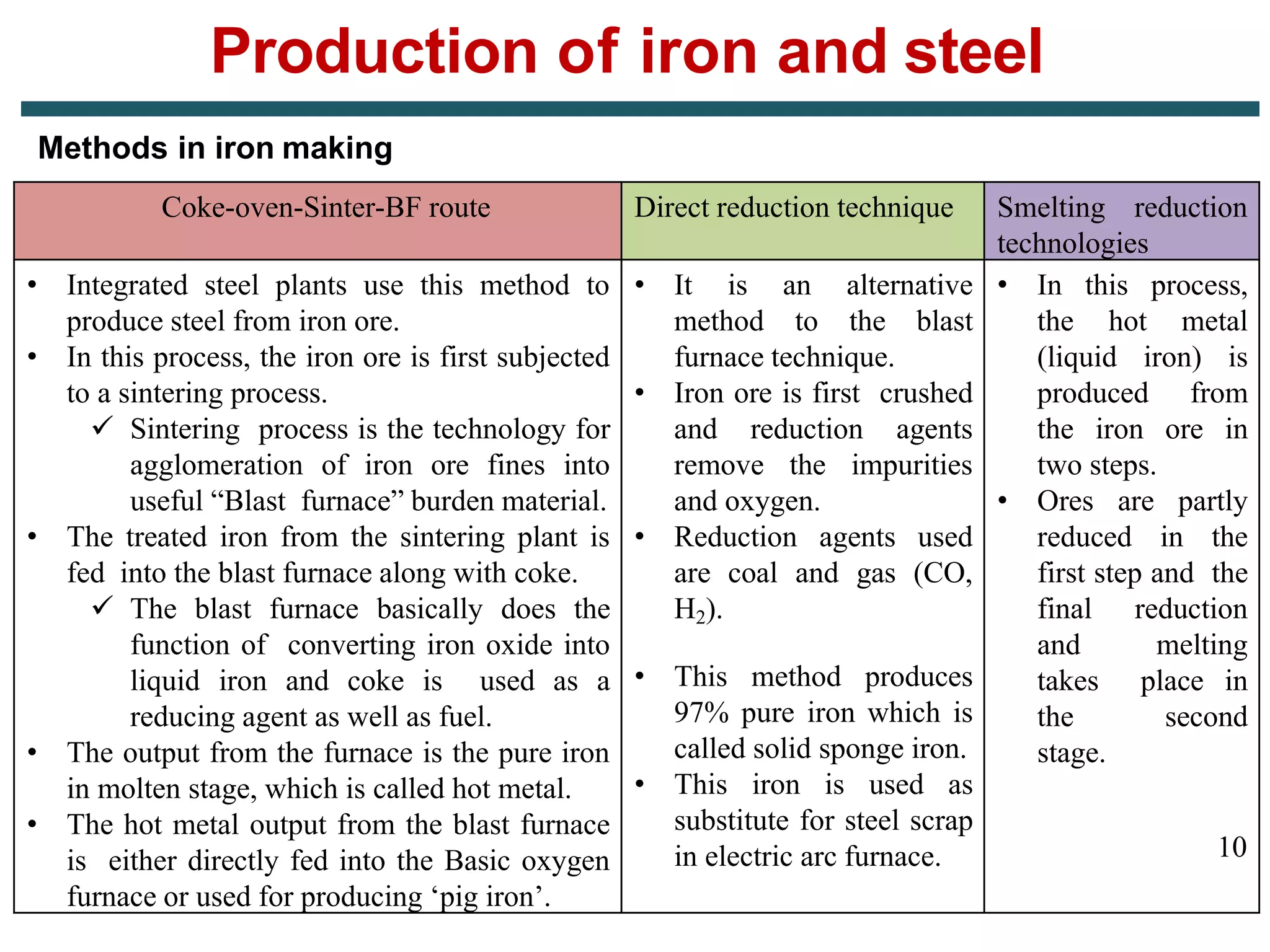
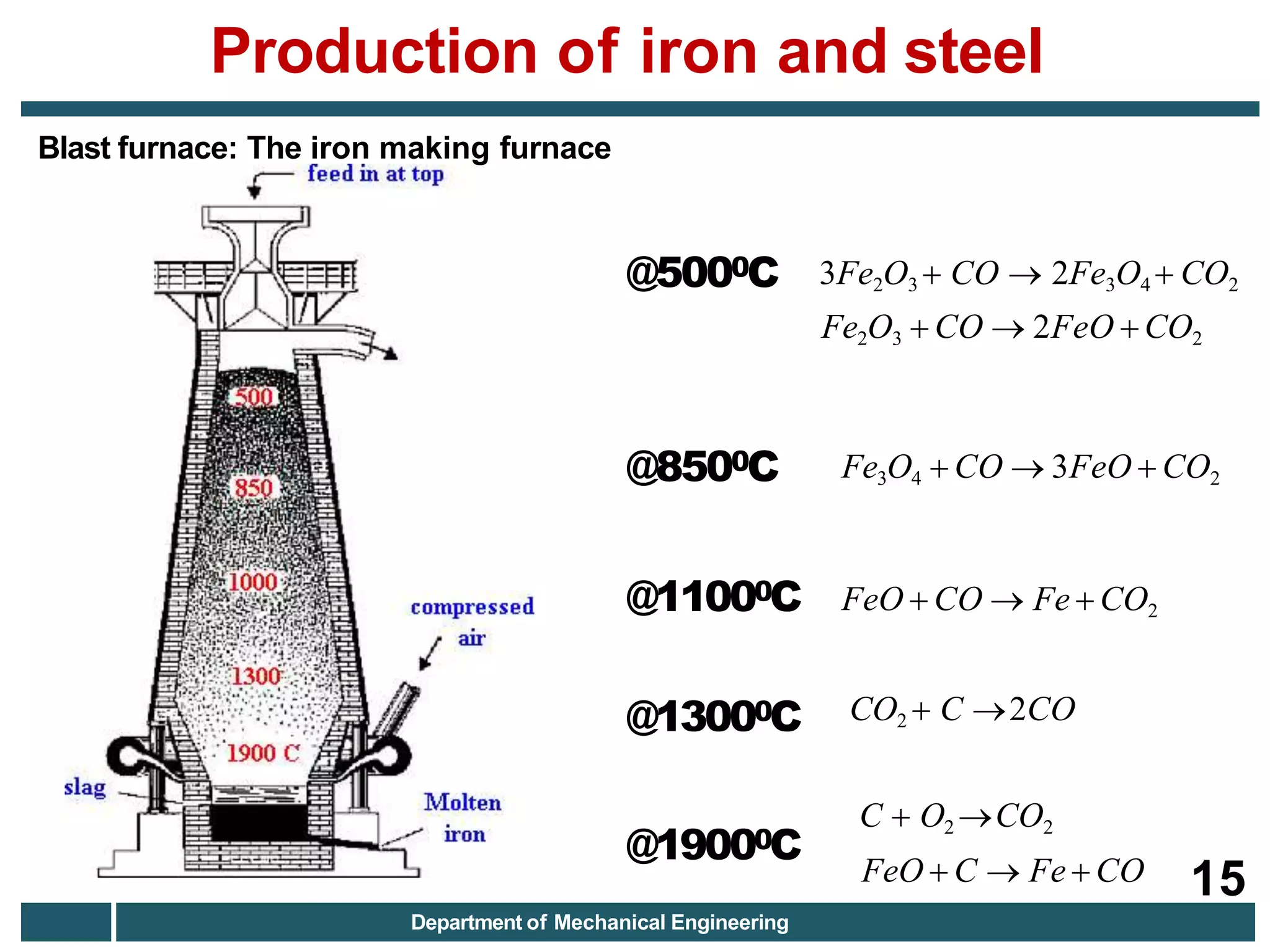
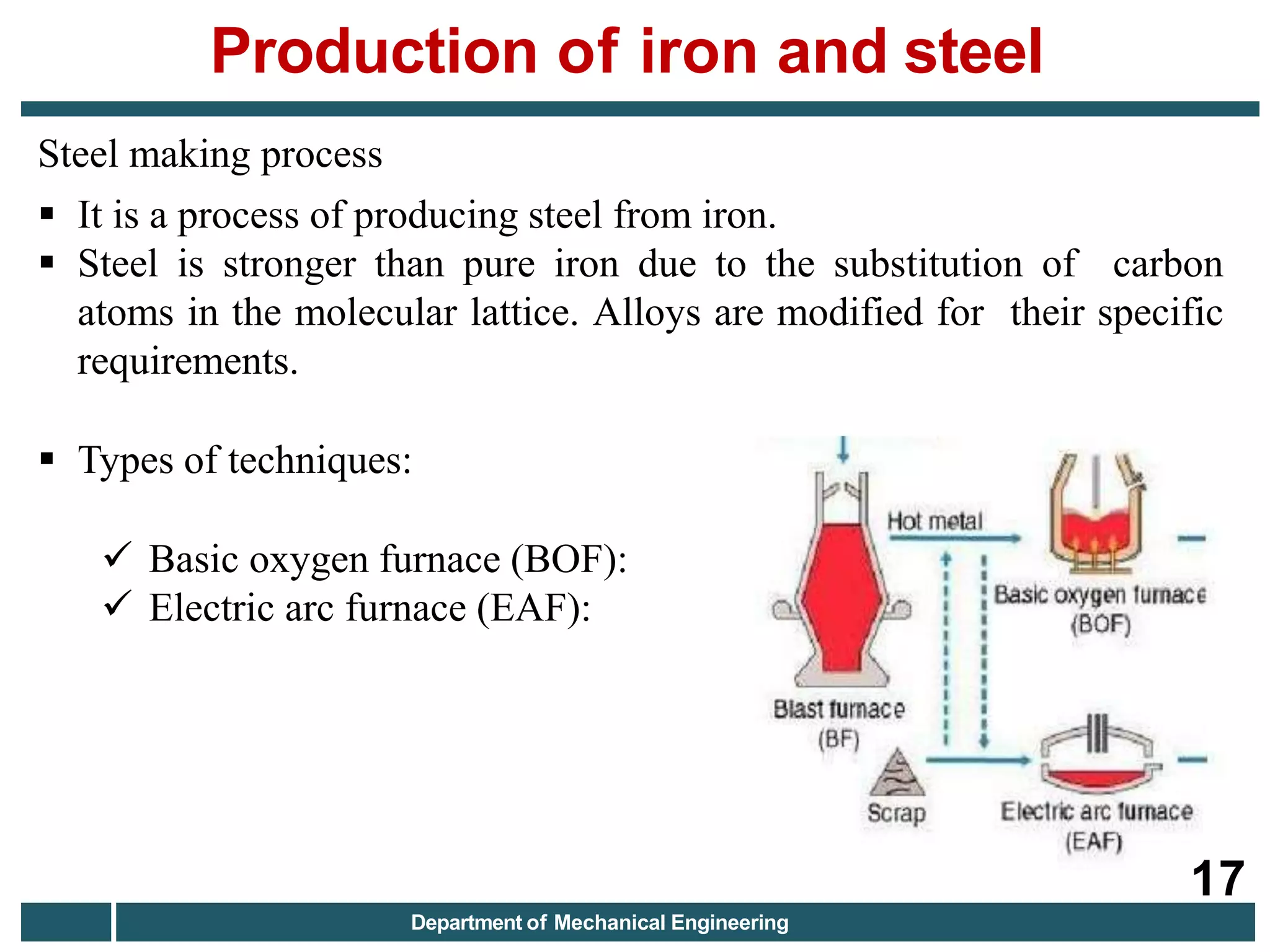
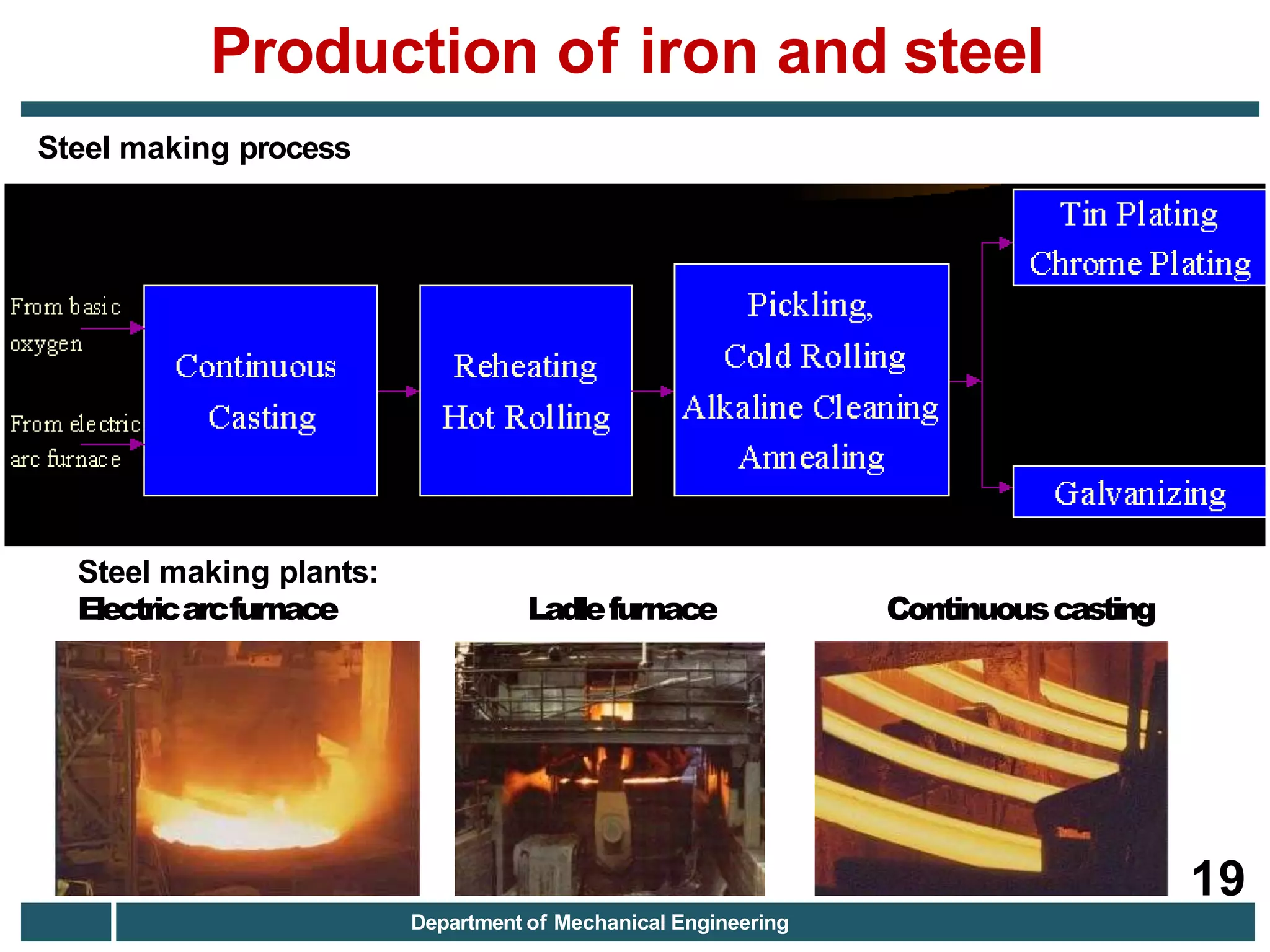
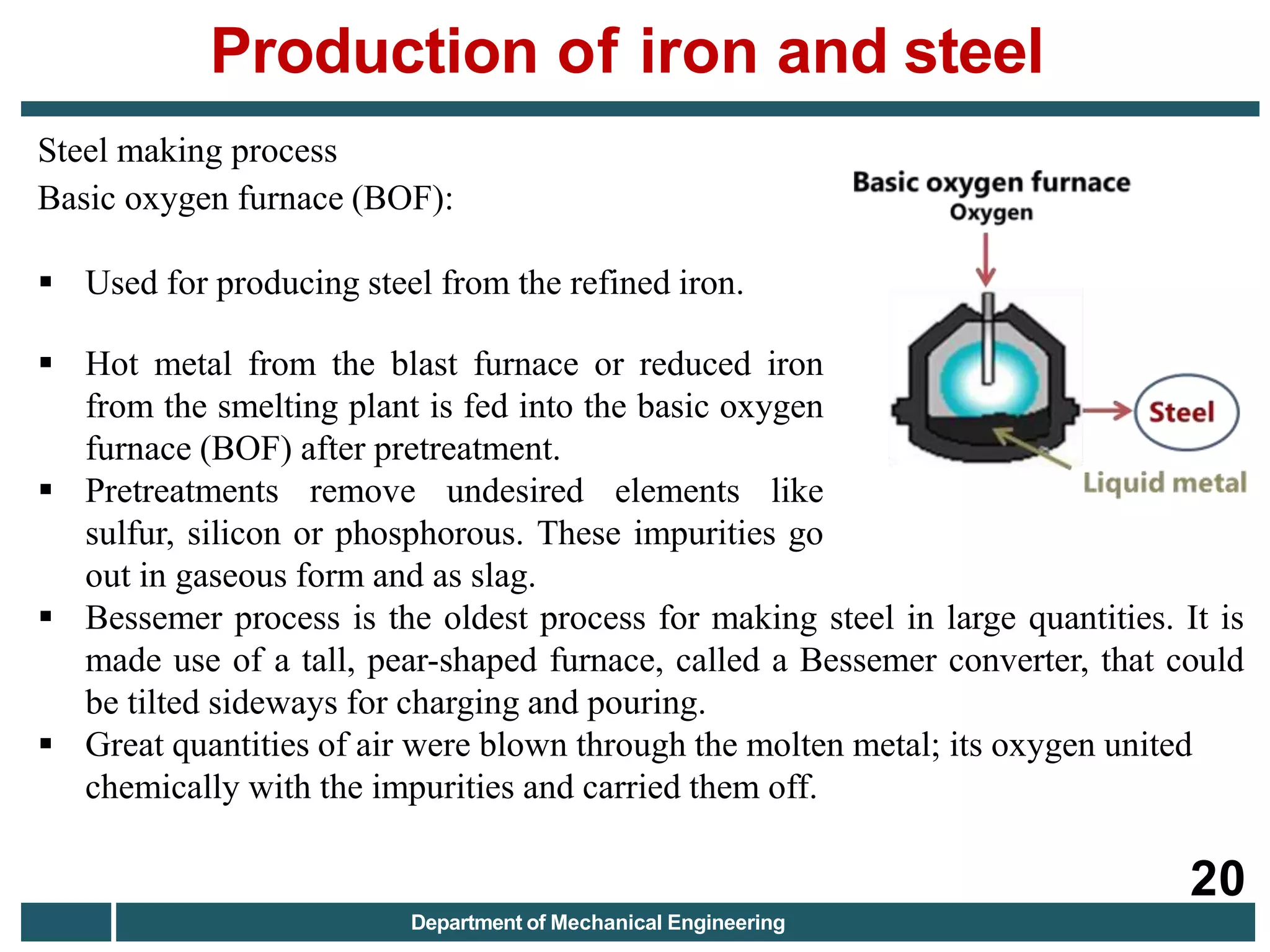
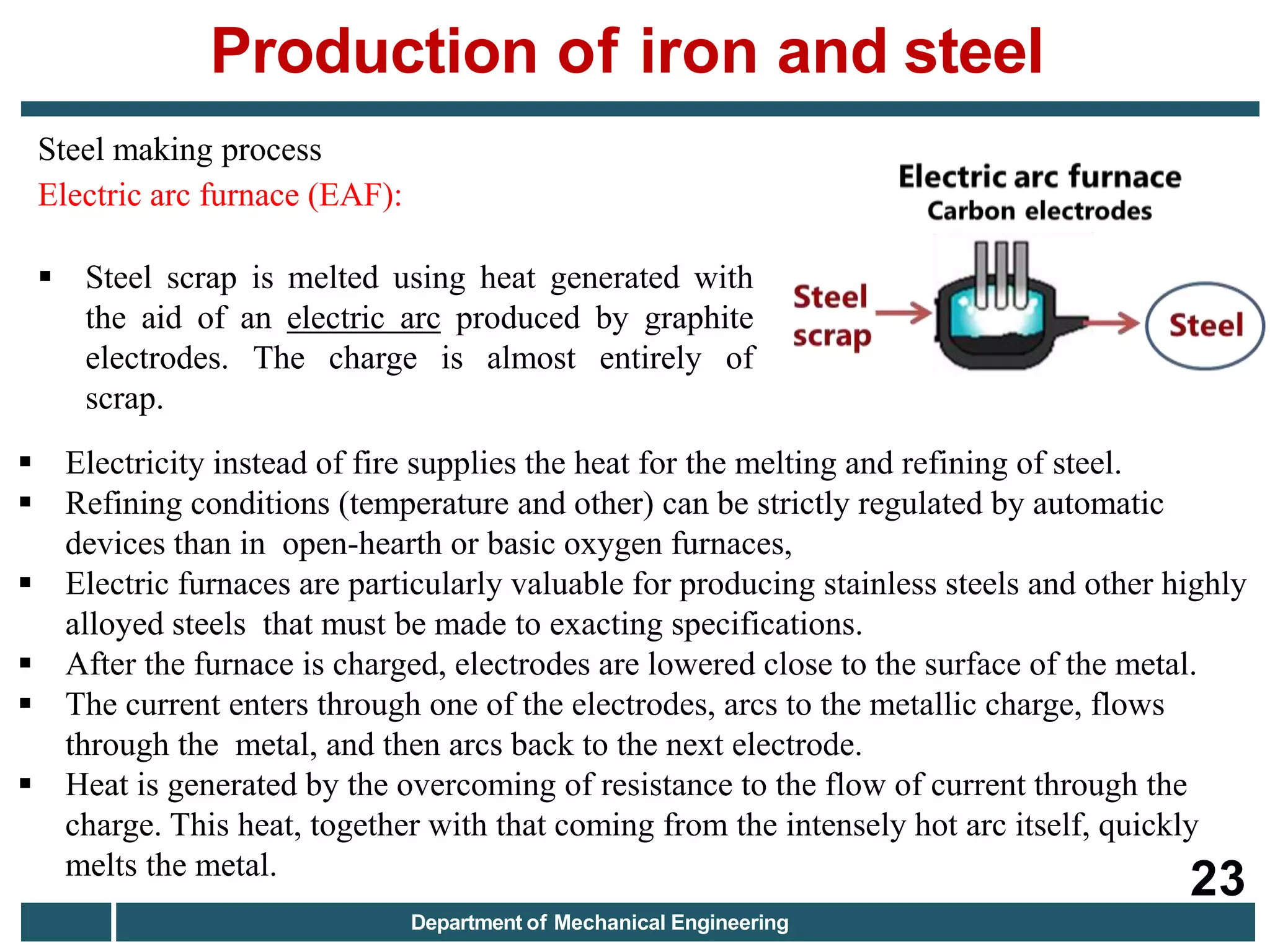
The document discusses the production of iron and steel, including the extraction of iron from iron ore in a blast furnace, the conversion of molten iron to steel through basic oxygen furnace or electric arc furnace processes, and finishing methods like continuous casting to produce final steel products like plates, coils, bars, pipes and other structural shapes. Key steps involve iron ore smelting, pig iron production, steel making through oxidation or electric processes, and continuous casting or ingot formation for further processing into finished goods.