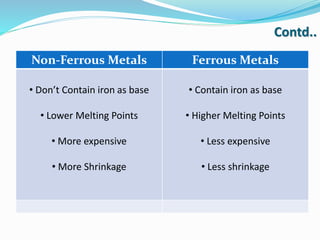
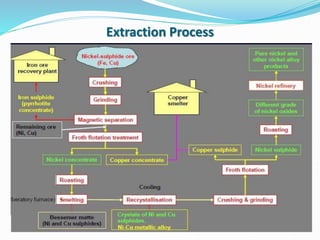
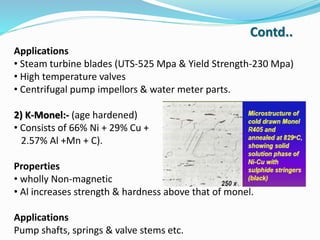
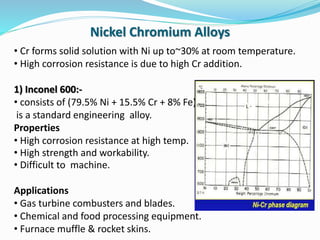
This document discusses non-ferrous metal nickel and its alloys. It begins with an introduction to nickel, noting its crystal structure, properties like hardness and ductility, and common uses. It then discusses various nickel alloys including commercially pure nickel, nickel-copper alloys, nickel-chromium alloys, and nickel-base superalloys. Specific alloys in each category like Monel and Inconel are described. Applications of different alloys in areas like turbines, chemicals and batteries are also mentioned. In conclusion, the document provides references used to compile the information presented.