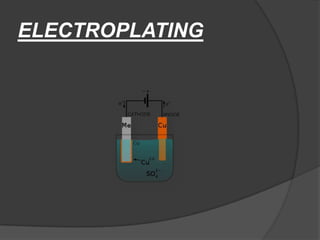
Metals are used widely in engineering. They are extracted from ores found naturally as compounds. The main types are ferrous (containing iron), non-ferrous (not containing iron), and alloys (mixtures). Important ferrous metals include mild steel, cast iron, and wrought iron. Metals must be processed and can be joined through methods like welding, brazing, and soldering to combat corrosion.