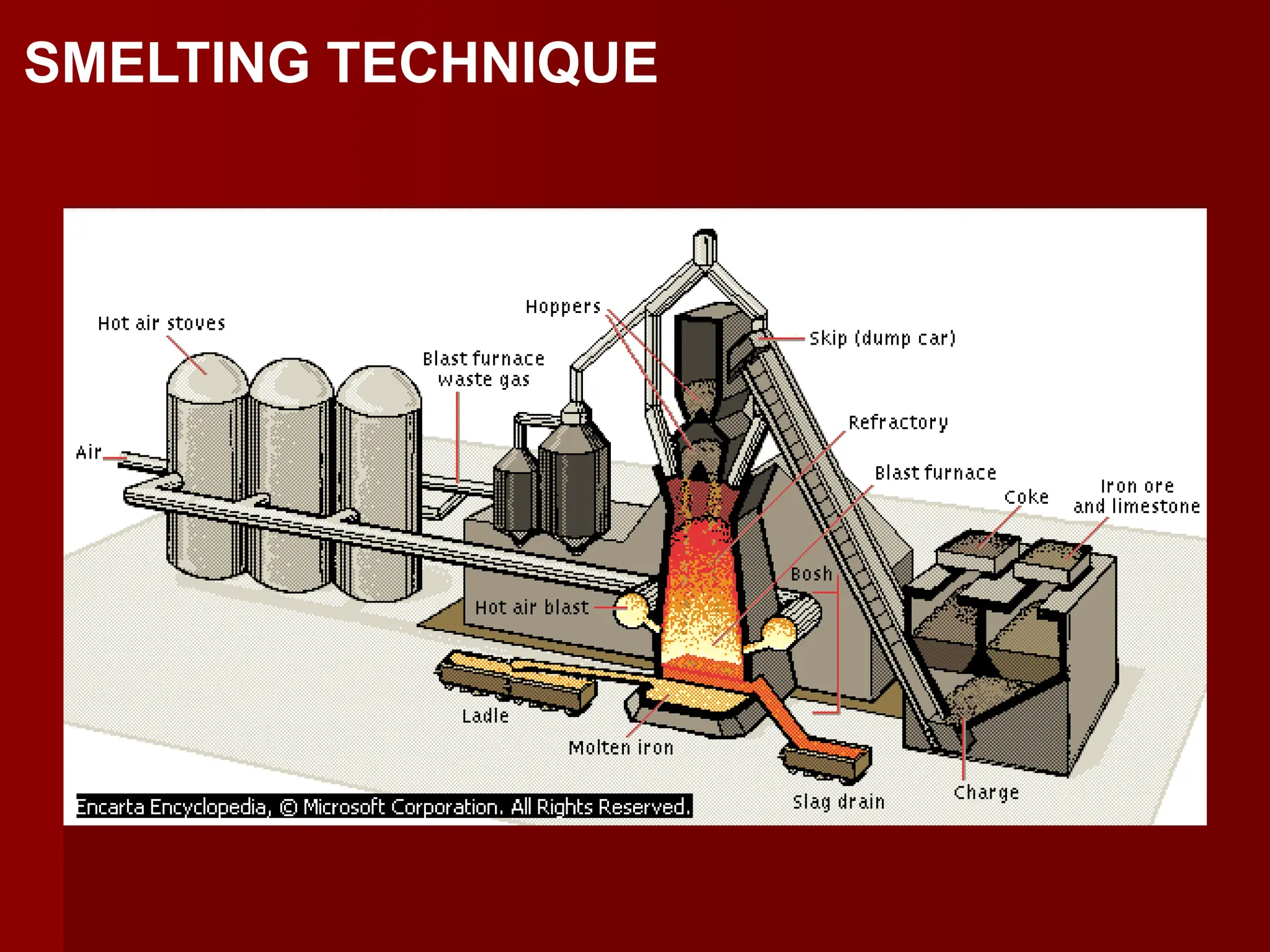
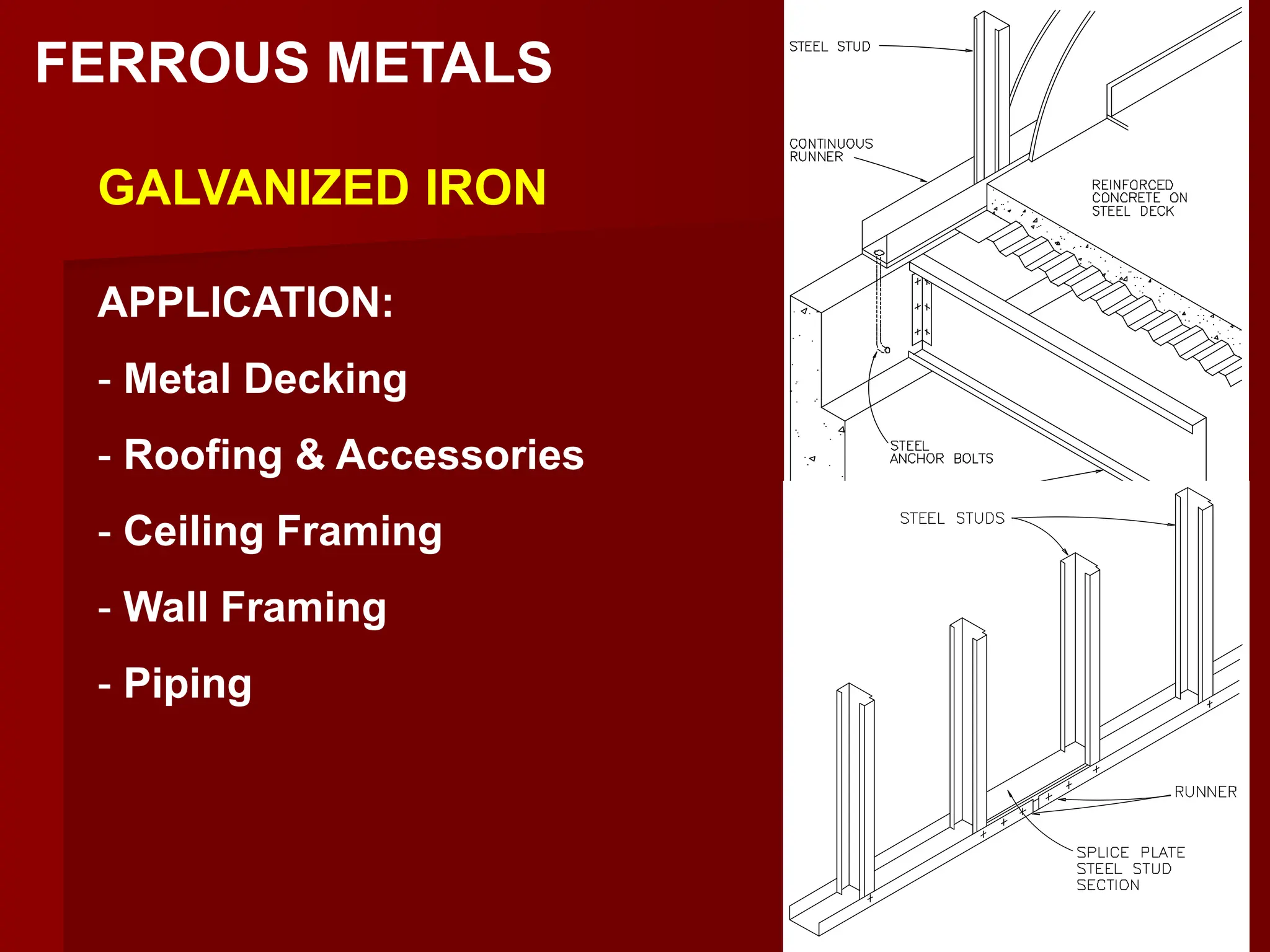
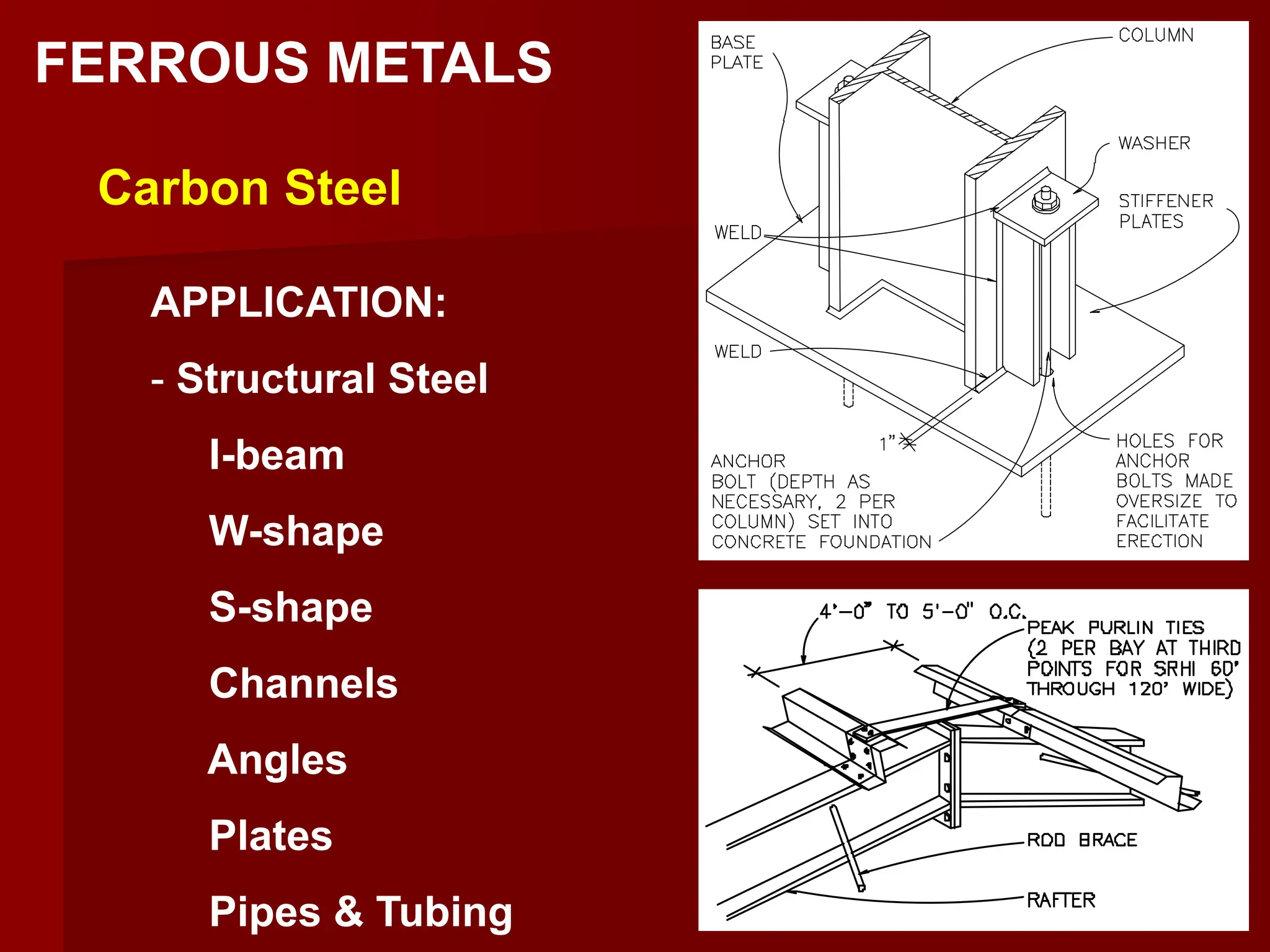
This document discusses ferrous and non-ferrous metals. Ferrous metals contain iron, such as cast iron, wrought iron, steel, and stainless steel. Non-ferrous metals contain little to no iron, including aluminum, bronze, brass, copper, and lead. The document provides details on the composition and applications of various metals.