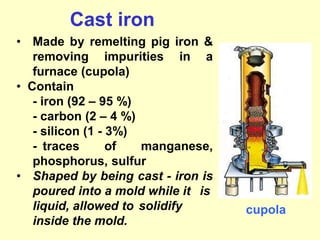
1. Metals are classified as ferrous, containing iron as the main constituent like steel and cast iron, or non-ferrous without iron.
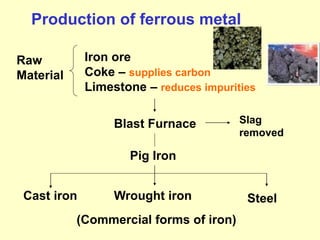
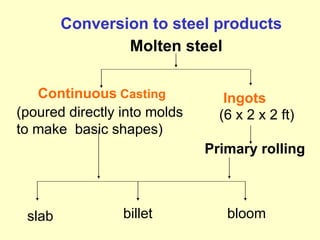
2. Iron ore, limestone, and coke are used to produce pig iron in a blast furnace. Pig iron is then processed to produce wrought iron or steel.
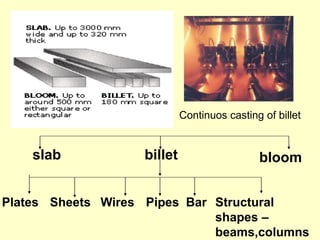
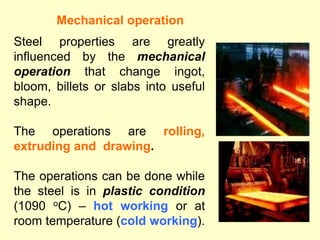
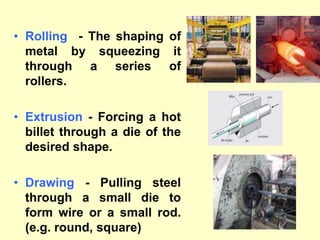
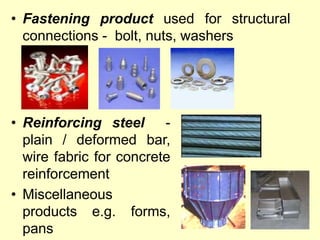
3. Steel has a variety of uses in construction and manufacturing due to its high strength, ductility, and ability to be cast and formed into different shapes. Its properties can be altered through adjusting carbon content, adding alloys, and heat treatment.