This document describes procedures for potentiometric titration experiments involving aspirin, vinegar, and sodium carbonate samples. Potentiometric titration uses a pH electrode and reference electrode connected to a pH meter to monitor pH changes during titration. For the aspirin experiment, an aspirin tablet is dissolved and titrated with sodium hydroxide while pH is recorded. The vinegar experiment involves titrating a vinegar sample with hydrochloric acid. For sodium carbonate, the sample produces two equivalence points when titrated with hydrochloric acid due to its carbonate ions. Data analysis involves calculating percent composition and errors from the titration curves and derivative plots.
![EXPERIMENT V
POTENTIOMETRIC TITRATION
These files are in Adobe Acrobat format, if you are using Netscape Navigator or Internet
Explorer and have Adobe Acrobat Reader installed (If you do not; Acrobat Reader can be
downloaded for free from Adobe) these files should open directly in your browser.
INTRODUCTION
Many Acid-Base titrations are difficult to accomplish using a visual indicator for one of several
reasons. Perhaps the analyst is color-blind to a particular indicator color change; there may not
be a suitable color change available for a particular type of titration or the solutions themselves
may be colored, opaque or turbid. It may be desired to automate a series of replicate
determinations. In such situations, potentiometric titration, using a glass hydronium ion selective
electrode, a suitable reference electrode and a sensitive potentiometer (a pH meter) may be
advantageous.
THEORY
Any acid-base titration may be conducted potentiometrically. Two electrodes, after calibration
[to relate potential in millivolts (mV) to a pH value] are immersed in a solution of the analyte.
One is an indicator electrode, selective for H3O+
and the other a stable reference electrode. The
potential difference, which after calibration is pH, is measured after the successive addition of
known increments of acid or base titrant.
When a potentiometric titration is being performed, interest is focused upon changes in the emf
of an electrolytic cell as a titrant of known concentration is added to a solution of unknown. The
method can be applied to all titrimetric reactions provided that the concentration of at least one
of the substances involved can be followed by means of a suitable indicator electrode. The
critical problem in a titration is to recognize the point at which the quantities of reacting species
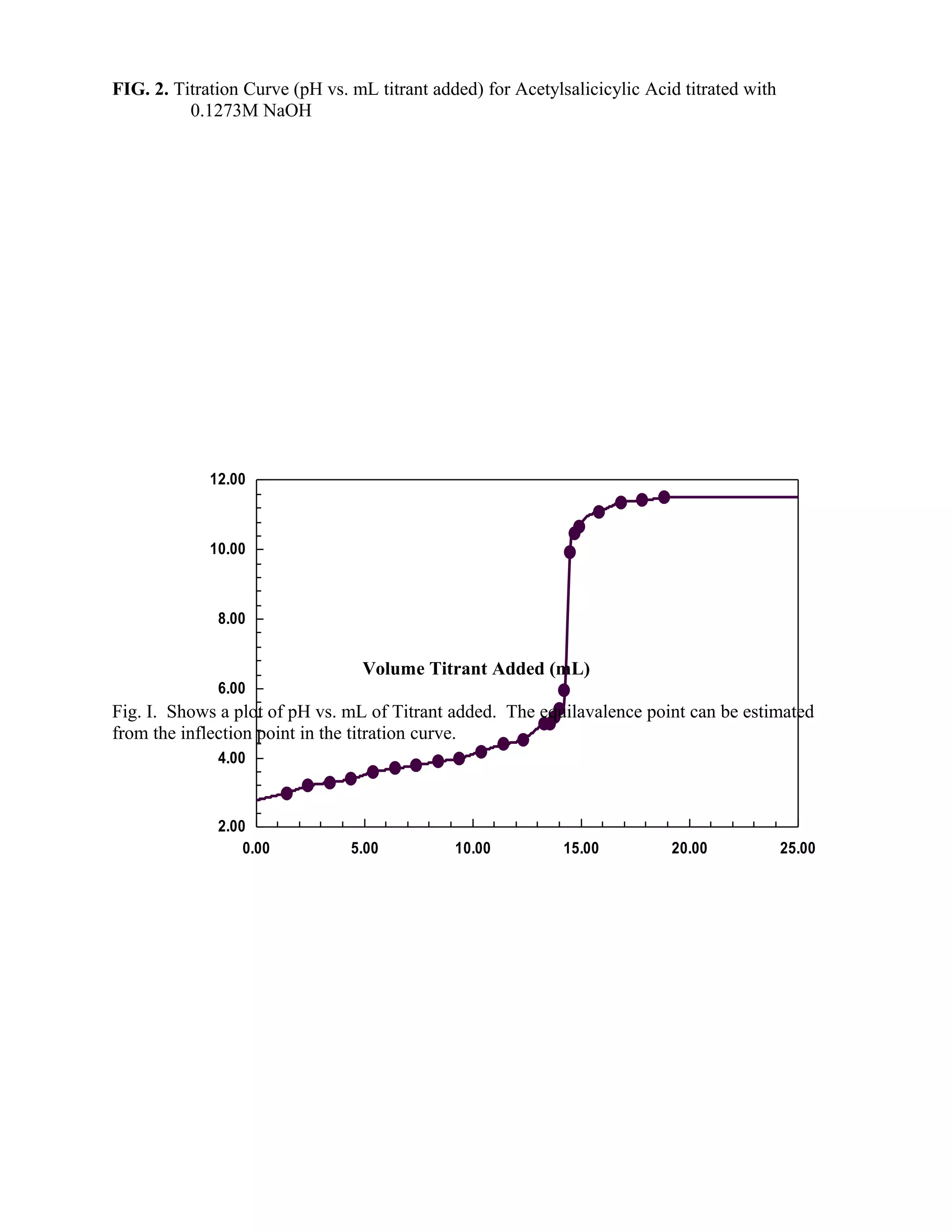
are present in equivalent amounts. The titration curve can be followed point by point, plotting as
ordinant, successive values of the cell emf (pH) vs the corresponding volume of titrant added. A
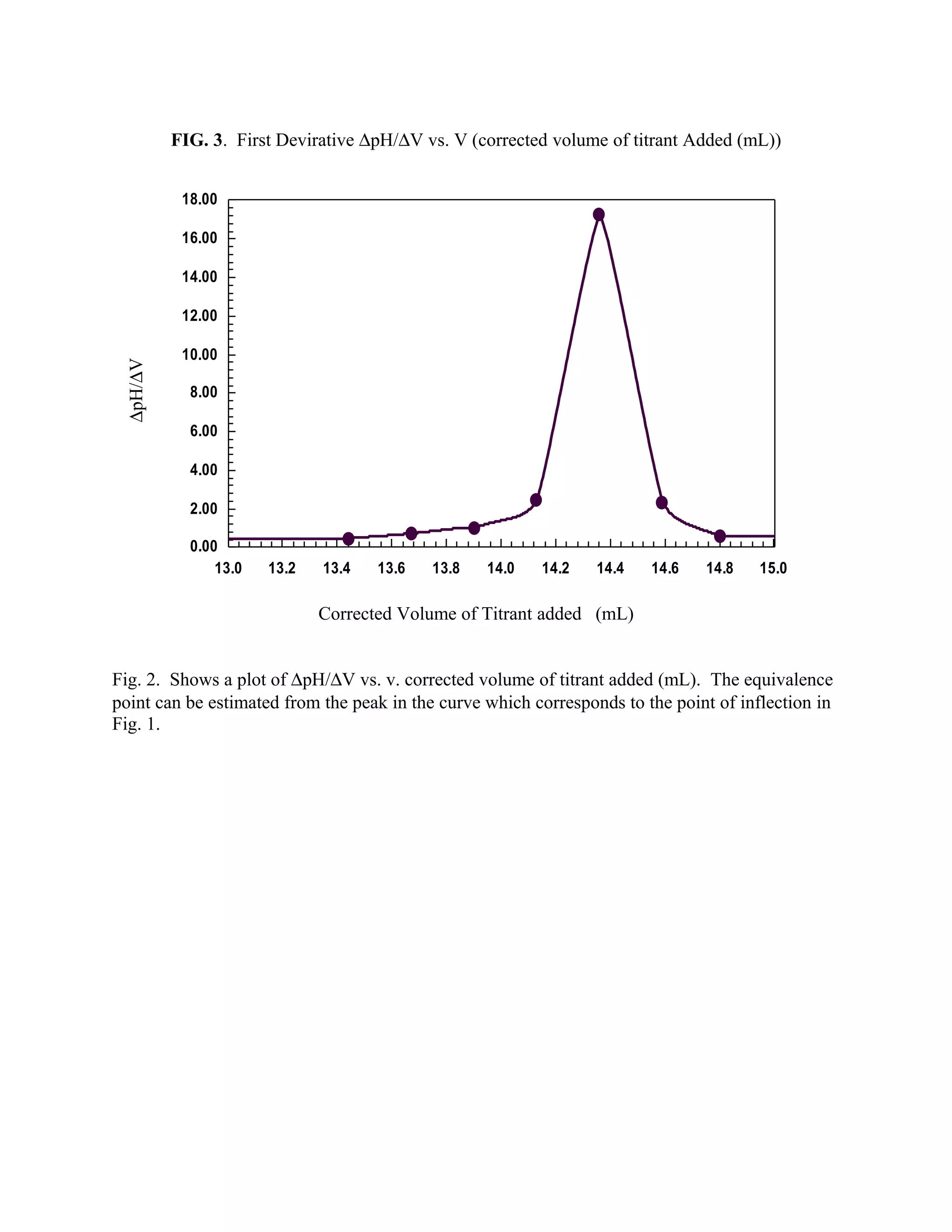
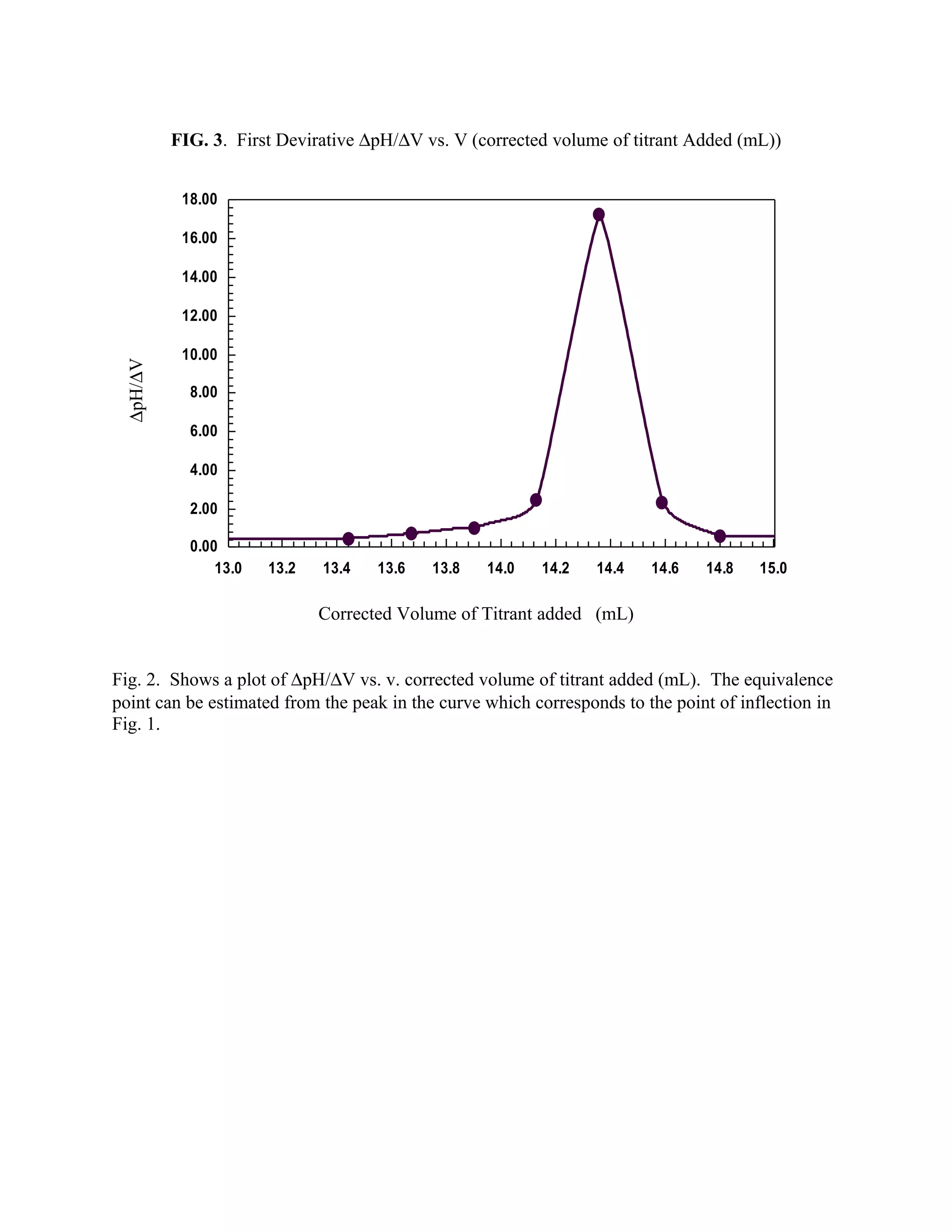
typical titration curve is presented in Figure 2. Figure 3 represent another method for
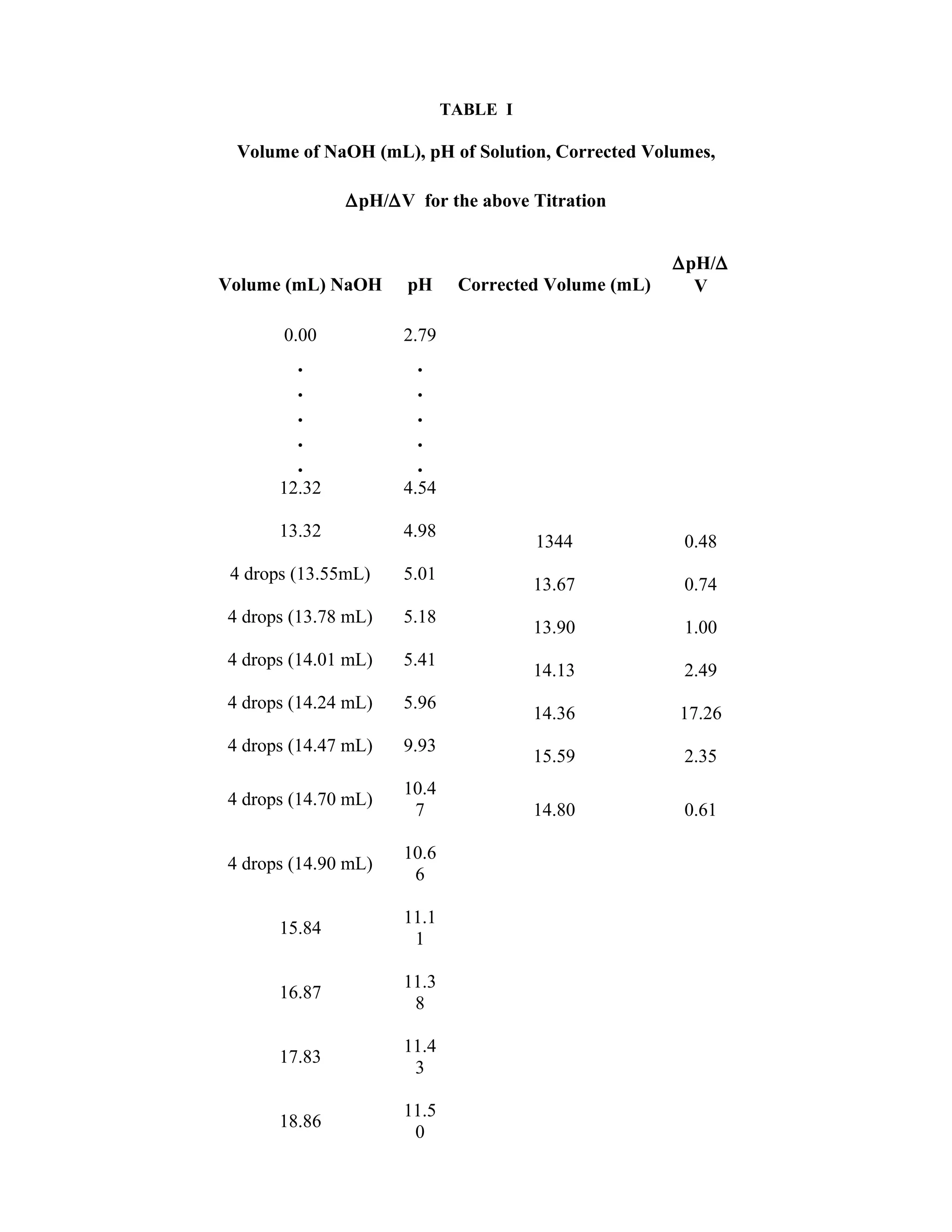
determining the equivalence point from the titration curve data. Table I, in Appendix I, presents
typical data obtained from a potentiometric titration.
The Reference Electrode
Most commonly, the reference electrode is the silver/silver chloride electrode. The
potential is based on the following equilibrium:
AgCl(s) + e →
←
Ag(s) + Cl-
(aq)
The half cell is:
Ag[(AgCl(Sat'd),KCl(xM)]
NOTE: Electrodes respond to the activity of the electroactive species in solution. However, as a practical
matter it is more convenient experimentally to use concentration. For this reason the discussion in this laboratory
experiment will be made in terms of concentration rather than the more correct activities.](https://image.slidesharecdn.com/experiment5chem210-130913220138-phpapp01/75/Experiment5-chem210-1-2048.jpg)
![The practical version of this electrode is a silver wire dipping into a saturated solution of
KCl; when fabricated this way its electrode potential is 0.199V (vs. Normal Hydrogen Electrode,
NHE) @ 25o
C. The potential is a function of temperature and the concentration of KCl in the
solution. Such an electrode is comparatively rugged, reliable, and inexpensive.
The Indicator Electrode
The heart of the glass electrode is a thin glass membrane, specially fabricated to preferentially
exchange H3O+
. The outside of the membrane is in contact with the analyte solution containing
the unknown [H3O+
]. The inside of the membrane contacts a hydrochloric acid solution of fixed
concentration. A silver wire, coated with AgCl dips into this solution; the other end of the wire
is connected to the measuring device. A combination glass electrode with silver/silver chloride
reference may be represented as shown in Figure 1 below:
FIG. 1. pH Electrode
The Mechanism of the Response
A change in hydronium ion concentration causes a change in composition of the glass membrane
due to an ion exchange process involving the solution and the membrane (see textbook for
details). A corresponding change in membrane potential, proportional to pH, is what is
measured. All other potentials are constant. In effect the membrane potential (variable) is
measured against two fixed potentials, the external reference and the internal reference, both
Ag/AgCl reference electrodes.
Potential difference is measured using a high impedance potentiometer. This high resistance
dictates a very small current flow.
Each glass electrode is different, due to the difficulty of reproducing the glass membrane; it is,
therefore, necessary to standardize the meter and electrode against at least two solutions of
accurately known pH. Such standard buffer solutions are available from many different
manufacturers.](https://image.slidesharecdn.com/experiment5chem210-130913220138-phpapp01/75/Experiment5-chem210-2-2048.jpg)