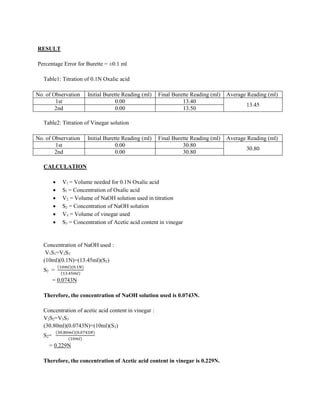
The document describes an experiment to determine the concentration of acetic acid in vinegar using an acid-base titration with sodium hydroxide. Vinegar and a standard solution of oxalic acid are each titrated with sodium hydroxide solution. The volume of sodium hydroxide used allows the concentration of the sodium hydroxide to be calculated. This concentration is then used along with the volume of sodium hydroxide used to titrate the vinegar sample to determine the concentration of acetic acid in the vinegar, which is found to be 0.229N.