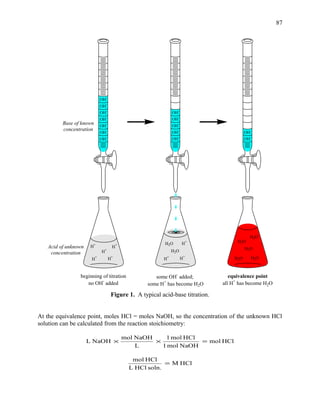
This document describes a procedure to determine the concentration of acetic acid in vinegar through acid-base titration. Vinegar samples of known volume will be titrated with a sodium hydroxide solution of known molarity. The collected data on titrant volume will be used to calculate the moles of acetic acid present and from that, the molarity and percentage by mass of acetic acid in the vinegar samples. Students will perform the titration, record observations, calculate results, and report the average molarity and percentage of acetic acid determined.