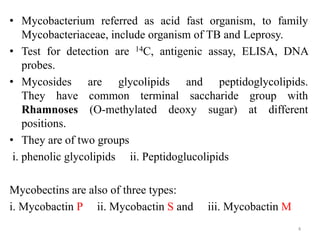
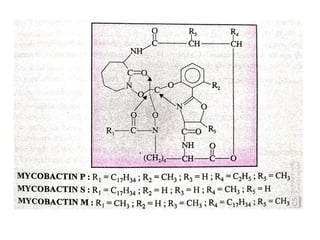
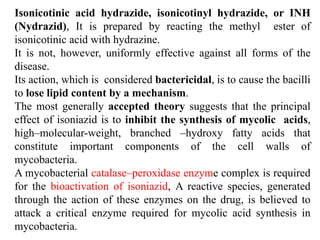
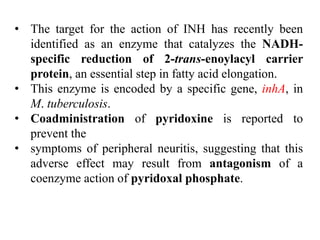
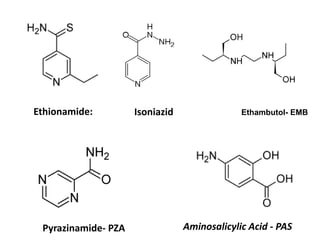
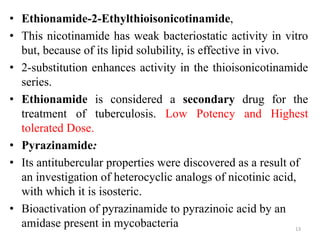
1. Tuberculosis remains a major global health problem, causing millions of deaths each year. New antitubercular agents are needed to combat drug resistance.
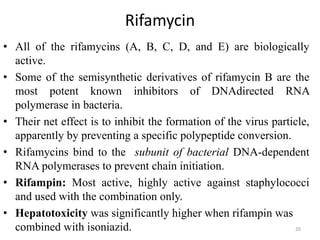
2. The document discusses the development of various classes of antitubercular agents, including synthetic drugs like isoniazid and rifampin, and antibiotics such as streptomycin. It covers their mechanisms of action, effectiveness, and toxicity.
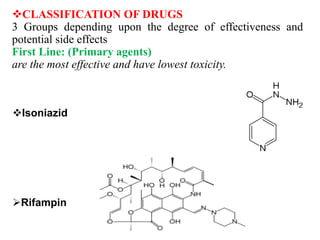
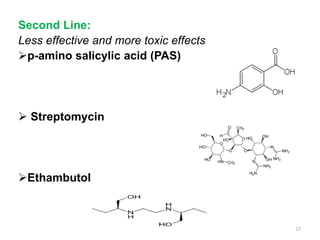
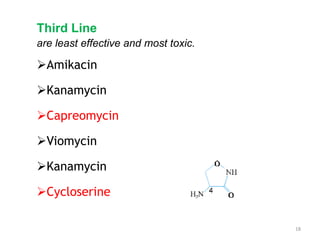
3. Classification of antitubercular drugs includes first-line agents that are most effective and least toxic, second-line alternatives for resistant cases, and third-line options that are least effective and most toxic. Developing improved drug combinations remains a priority area in tuberculosis treatment.