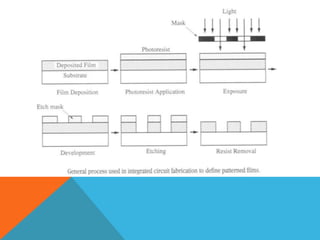
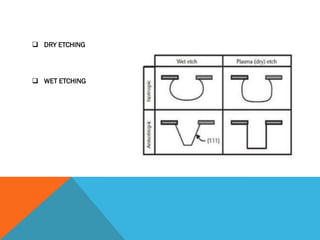
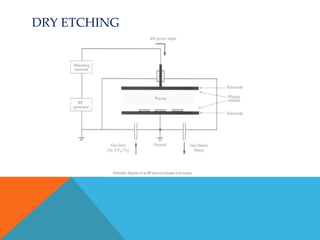
The document discusses etching processes in digital manufacturing, focusing on wet and dry etching techniques. It outlines the importance of etching in removing unwanted materials from semiconductor wafers and explains the advantages of plasma-based dry etching over traditional wet etching. Key factors such as etch selectivity, directionality, and the role of different materials and chemicals in the etching process are also explored.