Column Chromatography
•Download as DOCX, PDF•
20 likes•2,382 views
1. The document discusses column chromatography, which is a technique used to separate mixtures based on differential adsorption of substances onto a stationary phase. 2. Key aspects covered include the principle of column chromatography, types of adsorption (e.g. normal phase, reversed phase, ion exchange), factors that influence separation like nature of adsorbent and mobile phase, and applications like purification and analysis of mixtures. 3. Column chromatography works by flowing a mobile liquid phase over a packed stationary solid phase, allowing different substances in a mixture to migrate through the column at different rates based on their relative affinities for the mobile vs. stationary phases.
Report
Share
Report
Share
Recommended
Column chromatography

Presenting a presentation on the topic of Column chromatography with including basics of chromatography, principles, equations, graphs and data related to it.
Topics which covered in this ppt is
Principle of chromatography
classification of chromatography
partition coefficient
chromatogram
Resolution
plate theory
determination of N
band zone broadening
rate theory
https://www.linkedin.com/in/preeti-choudhary-266414182/
https://www.instagram.com/chaudharypreeti1997/
https://www.facebook.com/profile.php?id=100013419194533
https://twitter.com/preetic27018281
Please like, share, comment and follow.
stay connected
If any query then contact:
chaudharypreeti1997@gmail.com
Thanking-You
Preeti Choudhary
Lecture of chromatography

Chromatography is a technique used to separate mixtures into individual components. Paper chromatography is a type of chromatography that uses paper as the stationary phase. The mixture is applied to the paper and then placed in a developing chamber with the mobile phase solvent. As the solvent travels up the paper, the different components of the mixture separate based on how strongly they interact with the stationary and mobile phases. This creates discrete spots that can be analyzed to identify the components in the original mixture. Paper chromatography is a simple, inexpensive, and effective technique for separating and analyzing mixtures.
assignment on thin layer chromatography 

Thin layer chromatography (TLC) is a technique used to separate mixtures and identify compounds. It involves applying a sample to a thin layer of adsorbent material like silica gel coated on a plate. The plate is then placed in a developing chamber containing a mobile phase liquid. Each component in the sample travels differently through the stationary phase based on how strongly it interacts with the adsorbent material and mobile phase. The distance each compound travels allows it to be identified or quantified using its retention factor. TLC is a simple, fast, and inexpensive analytical technique used in research, purity testing, and separation of organic compounds.
Comparision of thin layer chromatography and paper chromatography

Thin layer chromatography (TLC) and paper chromatography are compared. TLC uses a silica gel coated plate as the stationary phase, while paper chromatography uses filter paper. TLC allows for sharper separation and is less time consuming than paper chromatography. TLC plates can also withstand higher temperatures and corrosive reagents compared to paper. TLC provides higher sensitivity for detection of fractions with less diffusion of spots.
Chromatography 

The document discusses the principles of chromatography. It describes how chromatography separates components in a mixture based on differences in their interactions with mobile and stationary phases. It discusses how Michael Tswett first demonstrated chromatography in 1903 and the key aspects of how it works. These include how retention time, partition coefficients, selectivity factors and efficiency parameters like plate number and height equivalent to a theoretical plate are used to characterize chromatographic separations.
Column chromatography 

A chromatographic technique used for the separation and purification of solids & liquids
principle is based on Adsorption
Ion exchange chromatography PPT.

Ion exchange chromatography is a process that separates ions and polar molecules based on their charge using an ion exchange resin. There are two main types of ion exchange - cation exchange which uses a negatively charged resin to adsorb positively charged proteins, and anion exchange which uses a positively charged resin to adsorb negatively charged proteins. The process involves equilibrating the resin, applying the sample mixture, then eluting the bound molecules by altering conditions such as pH or ionic strength to cause differential elution. Ion exchange chromatography is useful for purifying proteins and other charged biomolecules.
ION EXCHANGE CHROMATOGRAPHY

This document summarizes ion exchange chromatography. It describes how ion exchange chromatography works by exchanging ions between a charged stationary phase and sample ions in mobile phase. It discusses the different types of ion exchangers including resins, gels, and inorganic exchangers. Key factors that influence retention such as pH, ionic strength, and organic solvent content are also summarized. Finally, some common applications of ion exchange chromatography are highlighted such as separation of ions, water softening, and determination of analytes in various samples.
Recommended
Column chromatography

Presenting a presentation on the topic of Column chromatography with including basics of chromatography, principles, equations, graphs and data related to it.
Topics which covered in this ppt is
Principle of chromatography
classification of chromatography
partition coefficient
chromatogram
Resolution
plate theory
determination of N
band zone broadening
rate theory
https://www.linkedin.com/in/preeti-choudhary-266414182/
https://www.instagram.com/chaudharypreeti1997/
https://www.facebook.com/profile.php?id=100013419194533
https://twitter.com/preetic27018281
Please like, share, comment and follow.
stay connected
If any query then contact:
chaudharypreeti1997@gmail.com
Thanking-You
Preeti Choudhary
Lecture of chromatography

Chromatography is a technique used to separate mixtures into individual components. Paper chromatography is a type of chromatography that uses paper as the stationary phase. The mixture is applied to the paper and then placed in a developing chamber with the mobile phase solvent. As the solvent travels up the paper, the different components of the mixture separate based on how strongly they interact with the stationary and mobile phases. This creates discrete spots that can be analyzed to identify the components in the original mixture. Paper chromatography is a simple, inexpensive, and effective technique for separating and analyzing mixtures.
assignment on thin layer chromatography 

Thin layer chromatography (TLC) is a technique used to separate mixtures and identify compounds. It involves applying a sample to a thin layer of adsorbent material like silica gel coated on a plate. The plate is then placed in a developing chamber containing a mobile phase liquid. Each component in the sample travels differently through the stationary phase based on how strongly it interacts with the adsorbent material and mobile phase. The distance each compound travels allows it to be identified or quantified using its retention factor. TLC is a simple, fast, and inexpensive analytical technique used in research, purity testing, and separation of organic compounds.
Comparision of thin layer chromatography and paper chromatography

Thin layer chromatography (TLC) and paper chromatography are compared. TLC uses a silica gel coated plate as the stationary phase, while paper chromatography uses filter paper. TLC allows for sharper separation and is less time consuming than paper chromatography. TLC plates can also withstand higher temperatures and corrosive reagents compared to paper. TLC provides higher sensitivity for detection of fractions with less diffusion of spots.
Chromatography 

The document discusses the principles of chromatography. It describes how chromatography separates components in a mixture based on differences in their interactions with mobile and stationary phases. It discusses how Michael Tswett first demonstrated chromatography in 1903 and the key aspects of how it works. These include how retention time, partition coefficients, selectivity factors and efficiency parameters like plate number and height equivalent to a theoretical plate are used to characterize chromatographic separations.
Column chromatography 

A chromatographic technique used for the separation and purification of solids & liquids
principle is based on Adsorption
Ion exchange chromatography PPT.

Ion exchange chromatography is a process that separates ions and polar molecules based on their charge using an ion exchange resin. There are two main types of ion exchange - cation exchange which uses a negatively charged resin to adsorb positively charged proteins, and anion exchange which uses a positively charged resin to adsorb negatively charged proteins. The process involves equilibrating the resin, applying the sample mixture, then eluting the bound molecules by altering conditions such as pH or ionic strength to cause differential elution. Ion exchange chromatography is useful for purifying proteins and other charged biomolecules.
ION EXCHANGE CHROMATOGRAPHY

This document summarizes ion exchange chromatography. It describes how ion exchange chromatography works by exchanging ions between a charged stationary phase and sample ions in mobile phase. It discusses the different types of ion exchangers including resins, gels, and inorganic exchangers. Key factors that influence retention such as pH, ionic strength, and organic solvent content are also summarized. Finally, some common applications of ion exchange chromatography are highlighted such as separation of ions, water softening, and determination of analytes in various samples.
Ion exchange chromatography

Ion exchange chromatography separates mixtures of ions based on their affinity for ion exchange resins. There are two main types of resins - cation exchange resins that interact with positively charged ions, and anion exchange resins that interact with negatively charged ions. The mixture is passed through a column packed with the resin, and the different ions are selectively absorbed and eluted by changing the mobile phase, allowing separation of the individual components. Ion exchange chromatography has applications in water softening and purification, and separation of ions, sugars, and amino acids.
Column chromatography

This presentation contains all the topics related to column chromatography. That includes introduction, principle,apparatus, experimental aspects of column chromatography, application of column chromatography, advantage and disadvantage of column chromatography with reference.
Ion exchange chromatography

Ion exchange chromatography uses charged sites on a stationary phase to selectively retain ionized solutes from a mobile phase based on electrostatic attraction. Cation exchangers contain negatively charged groups that attract positively charged cations, while anion exchangers contain positively charged groups that attract negatively charged anions. Key factors that influence selectivity include ion charge, hydrated radius, and polarizability. Ion exchange chromatography has various applications including separation of ions, removal of interferents, water softening, and demineralization.
High performance liquid chromatography (HPLC)

High performance liquid chromatography (HPLC) head points:
HPLC Advantages Vs GC
Instrumentation
HPLC System
Separations
Mobile Phase Reservoirs
Degasser
Aim of Gradient system
High/Low pressure gradient system
HPLC Pump Criteria
HPLC Pumps: Types
Reciprocating Pumps
Sample introduction
Manual Injector
Auto Injector
HPLC Modes
The Mobile Phase
Hydrophobic interaction
Common reverse phase solvents
Detectors
https://www.linkedin.com/in/preeti-choudhary-266414182/
https://www.instagram.com/chaudharypreeti1997/
https://www.facebook.com/profile.php?id=100013419194533
https://twitter.com/preetic27018281
Please like, share, comment and follow.
stay connected
If any query then contact:
chaudharypreeti1997@gmail.com
Thanking-You
Preeti Choudhary
Classification of chromatography

Chromatography is a method of separating mixtures into individual components using a stationary and mobile phase. There are several types depending on the physical state of the phases and interaction between the phases and components. Liquid chromatography uses a liquid mobile phase passing through a solid or liquid stationary phase to separate components. Gas chromatography uses a gas mobile phase to separate volatile components. Size exclusion and ion exchange chromatography separate based on molecular size or charge.
1.column chromatography

Column chromatography is a technique used to separate mixtures based on differences in how components interact with a stationary and mobile phase. It involves passing a liquid mobile phase through a column containing a packed stationary solid phase. Components in the mixture are separated as they move through the column at different rates depending on how strongly they interact with each phase. Column chromatography has various applications including separation, purification, and isolation of compounds from mixtures like plant extracts.
Column chromatography ganesh

Column chromatography is a solid-liquid technique used to separate mixtures. It works by having different affinities of compounds for the stationary and mobile phases. The stationary phase is typically silica gel or alumina, and the mobile phase is selected based on the compounds' polarities. Factors like column length and diameter, adsorbent particle size, and elution mode affect the separation efficiency. Compounds are detected and collected as they exit the column based on their retention times. Column chromatography is useful for purification, isolation of compounds, and analytical applications.
HPLC (HIGH PERFORMANCE LIQUID CHROMATOGRAPHY)

This document provides an overview of high performance liquid chromatography (HPLC). It discusses the history and development of chromatography. The major components of an HPLC system are described, including pumps, detectors, columns, and recorders. The main separation modes are explained, including reversed-phase, normal-phase, ion exchange, and size exclusion chromatography. Parameters for method development and validation are outlined. Applications of HPLC in qualitative and quantitative analysis are also summarized.
Paper chromatography

The document discusses the process and history of paper chromatography. It begins with an introduction to chromatography and its use in separating mixtures. It then covers the history from early experiments in the 1860s to modern developments. The main types and techniques of paper chromatography are explained, including the use of a stationary phase, mobile phase, and capillary action to separate components by affinity and travel distance. Key steps like sample application and developing the paper strip are outlined.
Gas chromatography 

Introduction to gas Chromatography
,Principle of gas chromatography
Instrumentation of gas Chromatography
Type of detectors of gas chromatography
Advantages of gas chromatography
Disadvantages of gas chromatography
Applications of gas chromatography
Theories of chromatography

There are two theories that explain chromatography: plate theory and rate theory. Plate theory, developed in 1941, views the column as divided into theoretical plates where analytes completely equilibrate between the stationary and mobile phases. Rate theory, proposed in 1956, accounts for the dynamics of separation. Greater separation occurs with more theoretical plates and smaller plate height. The number of theoretical plates can be calculated using methods like half-height or USP, and depends on factors like column length, particle size, and retention time.
Silica Gel | Aluminium Oxide Column chroamtography

Buy Silica Gel Powder for Silica Gel, Aluminium Oxide, Paper and Flash Column Chromatography us in Pharmaceutical Industries – Bulk Drugs & API, Nutraceuticals, Herbal Extracts products manufacturers, Research Laboratories, Laboratories Chemical Repackaging, Contract Research Laboratories. Column Chromatography is one of the most useful methods for purification & separation (Isolation) of individual desire compound from mixture of unwanted compounds.
Adsorption Chromatography Assignment.pdf

Adsorption chromatography is a technique for separating components in a mixture based on differential adsorption of the components onto a stationary solid phase. It works by passing a mobile liquid or gas phase over an adsorbent stationary phase in a column, which causes components to separate as they are differentially retained on the surface of the adsorbent. Common types include thin layer chromatography, paper chromatography, and column chromatography. Adsorption chromatography has various applications such as separating amino acids, isolating antibiotics, and identifying carbohydrates.
Paper chromatography

Paper chromatography is partition chromatography in which solutes distribute between two liquid phases(water in paper and eluting solvent)
Spectrophotometry & beer's law

This document describes spectrophotometry and Beer's law. It discusses how spectrophotometers work by measuring the absorption of light by chemical substances over various wavelengths. It then describes two experiments conducted - the first determined the maximum absorbance wavelengths of different colored solutions, the second used Beer's law to calculate the concentrations of green solutions from their measured absorbances. The results showed that maximum absorbance correlated with complementary colors and absorbance increased with concentration as predicted by Beer's law.
Flash chromatography

Flash chromatography is a technique that uses compressed gas to rapidly push solvent through a column packed with adsorbent material like silica gel. This allows for faster separations compared to traditional gravity chromatography. The document discusses the principles, common solvent systems, packing columns, instrumentation used like pumps, fraction collectors, and detectors, and applications of flash chromatography.
Ion exchange chromatography -SlideShare

This ppt provide a good knowledge about ion exchange chromatography. I think this is very helpful for you .Here i have tried to explain a best way and simple method so guys you all enjoy this and gain your knowledge. And wish for me to provide more pptx for you all .at the end i want your experience give me suggestion if i made any mistake thank you .
Chromatography- Paper chromatography pdf

This document provides information about chromatography and paper chromatography. It defines chromatography as a technique used to purify and separate substances using their differential distribution between two phases, one stationary and one mobile. Paper chromatography is described as a type of planar chromatography where the stationary phase is supported on filter paper and the mobile phase moves through capillary action. Key aspects of paper chromatography covered include the principle, factors affecting separation, and different techniques such as ascending and descending development.
HPTLC

This document discusses high performance thin layer chromatography (HPTLC). It begins with an introduction and overview of HPTLC principles, instrumentation, differences from TLC, and application steps. The document then provides more details on the HPTLC instrumentation components, factors affecting separation, common stationary and mobile phases used, and application techniques. It concludes by discussing detection methods and some key applications of HPTLC in pharmaceuticals, food analysis, clinical studies, and forensics.
Theory of high performance liquid chromatography ppt

This document provides an overview of the theory of high performance liquid chromatography (HPLC). It discusses key concepts such as the retention factor (k), which is a measure of how long a compound is retained on the column. Selectivity (α) refers to the ability to distinguish between sample components, and is calculated as a ratio of the k values. Resolution (Rs) is the most important measure of separation, and depends on factors like k, α, and the number of theoretical plates (N). N is a measure of column efficiency, and the height equivalent of a theoretical plate (HETP) describes efficiency. The document outlines how these parameters can be optimized to improve separation and resolution.
Applied Pharmacognosy | Chromatography

Principles of chromatography
Chromatography’ is an analytical technique commonly used for separating a mixture of chemical substances into its individual components, so that the individual components can be thoroughly analyzed. There are many types of chromatography e.g., liquid chromatography, gas chromatography, ion-exchange chromatography, affinity chromatography, but all of these employ the same basic principles.
Chromatography is a separation technique that every organic chemist and biochemist is familiar with. I, myself, being an organic chemist, have routinely carried out chromatographic separations of a variety of mixture of compounds in the lab. In fact, I was leafing through my research slides and came across a pictorial representation of an actual chromatographic separation that I had carried out in the lab
DSE-2, ANALYTICAL METHODS -Ch-II.pptx

The document discusses various analytical chromatography techniques. It describes chromatography as separating components through distribution between two immiscible phases, with one stationary and one mobile. The document outlines different types of chromatography including column chromatography, thin layer chromatography, gas chromatography, and ion exchange chromatography. It discusses the principles, techniques, and efficiency of these analytical methods.
More Related Content
What's hot
Ion exchange chromatography

Ion exchange chromatography separates mixtures of ions based on their affinity for ion exchange resins. There are two main types of resins - cation exchange resins that interact with positively charged ions, and anion exchange resins that interact with negatively charged ions. The mixture is passed through a column packed with the resin, and the different ions are selectively absorbed and eluted by changing the mobile phase, allowing separation of the individual components. Ion exchange chromatography has applications in water softening and purification, and separation of ions, sugars, and amino acids.
Column chromatography

This presentation contains all the topics related to column chromatography. That includes introduction, principle,apparatus, experimental aspects of column chromatography, application of column chromatography, advantage and disadvantage of column chromatography with reference.
Ion exchange chromatography

Ion exchange chromatography uses charged sites on a stationary phase to selectively retain ionized solutes from a mobile phase based on electrostatic attraction. Cation exchangers contain negatively charged groups that attract positively charged cations, while anion exchangers contain positively charged groups that attract negatively charged anions. Key factors that influence selectivity include ion charge, hydrated radius, and polarizability. Ion exchange chromatography has various applications including separation of ions, removal of interferents, water softening, and demineralization.
High performance liquid chromatography (HPLC)

High performance liquid chromatography (HPLC) head points:
HPLC Advantages Vs GC
Instrumentation
HPLC System
Separations
Mobile Phase Reservoirs
Degasser
Aim of Gradient system
High/Low pressure gradient system
HPLC Pump Criteria
HPLC Pumps: Types
Reciprocating Pumps
Sample introduction
Manual Injector
Auto Injector
HPLC Modes
The Mobile Phase
Hydrophobic interaction
Common reverse phase solvents
Detectors
https://www.linkedin.com/in/preeti-choudhary-266414182/
https://www.instagram.com/chaudharypreeti1997/
https://www.facebook.com/profile.php?id=100013419194533
https://twitter.com/preetic27018281
Please like, share, comment and follow.
stay connected
If any query then contact:
chaudharypreeti1997@gmail.com
Thanking-You
Preeti Choudhary
Classification of chromatography

Chromatography is a method of separating mixtures into individual components using a stationary and mobile phase. There are several types depending on the physical state of the phases and interaction between the phases and components. Liquid chromatography uses a liquid mobile phase passing through a solid or liquid stationary phase to separate components. Gas chromatography uses a gas mobile phase to separate volatile components. Size exclusion and ion exchange chromatography separate based on molecular size or charge.
1.column chromatography

Column chromatography is a technique used to separate mixtures based on differences in how components interact with a stationary and mobile phase. It involves passing a liquid mobile phase through a column containing a packed stationary solid phase. Components in the mixture are separated as they move through the column at different rates depending on how strongly they interact with each phase. Column chromatography has various applications including separation, purification, and isolation of compounds from mixtures like plant extracts.
Column chromatography ganesh

Column chromatography is a solid-liquid technique used to separate mixtures. It works by having different affinities of compounds for the stationary and mobile phases. The stationary phase is typically silica gel or alumina, and the mobile phase is selected based on the compounds' polarities. Factors like column length and diameter, adsorbent particle size, and elution mode affect the separation efficiency. Compounds are detected and collected as they exit the column based on their retention times. Column chromatography is useful for purification, isolation of compounds, and analytical applications.
HPLC (HIGH PERFORMANCE LIQUID CHROMATOGRAPHY)

This document provides an overview of high performance liquid chromatography (HPLC). It discusses the history and development of chromatography. The major components of an HPLC system are described, including pumps, detectors, columns, and recorders. The main separation modes are explained, including reversed-phase, normal-phase, ion exchange, and size exclusion chromatography. Parameters for method development and validation are outlined. Applications of HPLC in qualitative and quantitative analysis are also summarized.
Paper chromatography

The document discusses the process and history of paper chromatography. It begins with an introduction to chromatography and its use in separating mixtures. It then covers the history from early experiments in the 1860s to modern developments. The main types and techniques of paper chromatography are explained, including the use of a stationary phase, mobile phase, and capillary action to separate components by affinity and travel distance. Key steps like sample application and developing the paper strip are outlined.
Gas chromatography 

Introduction to gas Chromatography
,Principle of gas chromatography
Instrumentation of gas Chromatography
Type of detectors of gas chromatography
Advantages of gas chromatography
Disadvantages of gas chromatography
Applications of gas chromatography
Theories of chromatography

There are two theories that explain chromatography: plate theory and rate theory. Plate theory, developed in 1941, views the column as divided into theoretical plates where analytes completely equilibrate between the stationary and mobile phases. Rate theory, proposed in 1956, accounts for the dynamics of separation. Greater separation occurs with more theoretical plates and smaller plate height. The number of theoretical plates can be calculated using methods like half-height or USP, and depends on factors like column length, particle size, and retention time.
Silica Gel | Aluminium Oxide Column chroamtography

Buy Silica Gel Powder for Silica Gel, Aluminium Oxide, Paper and Flash Column Chromatography us in Pharmaceutical Industries – Bulk Drugs & API, Nutraceuticals, Herbal Extracts products manufacturers, Research Laboratories, Laboratories Chemical Repackaging, Contract Research Laboratories. Column Chromatography is one of the most useful methods for purification & separation (Isolation) of individual desire compound from mixture of unwanted compounds.
Adsorption Chromatography Assignment.pdf

Adsorption chromatography is a technique for separating components in a mixture based on differential adsorption of the components onto a stationary solid phase. It works by passing a mobile liquid or gas phase over an adsorbent stationary phase in a column, which causes components to separate as they are differentially retained on the surface of the adsorbent. Common types include thin layer chromatography, paper chromatography, and column chromatography. Adsorption chromatography has various applications such as separating amino acids, isolating antibiotics, and identifying carbohydrates.
Paper chromatography

Paper chromatography is partition chromatography in which solutes distribute between two liquid phases(water in paper and eluting solvent)
Spectrophotometry & beer's law

This document describes spectrophotometry and Beer's law. It discusses how spectrophotometers work by measuring the absorption of light by chemical substances over various wavelengths. It then describes two experiments conducted - the first determined the maximum absorbance wavelengths of different colored solutions, the second used Beer's law to calculate the concentrations of green solutions from their measured absorbances. The results showed that maximum absorbance correlated with complementary colors and absorbance increased with concentration as predicted by Beer's law.
Flash chromatography

Flash chromatography is a technique that uses compressed gas to rapidly push solvent through a column packed with adsorbent material like silica gel. This allows for faster separations compared to traditional gravity chromatography. The document discusses the principles, common solvent systems, packing columns, instrumentation used like pumps, fraction collectors, and detectors, and applications of flash chromatography.
Ion exchange chromatography -SlideShare

This ppt provide a good knowledge about ion exchange chromatography. I think this is very helpful for you .Here i have tried to explain a best way and simple method so guys you all enjoy this and gain your knowledge. And wish for me to provide more pptx for you all .at the end i want your experience give me suggestion if i made any mistake thank you .
Chromatography- Paper chromatography pdf

This document provides information about chromatography and paper chromatography. It defines chromatography as a technique used to purify and separate substances using their differential distribution between two phases, one stationary and one mobile. Paper chromatography is described as a type of planar chromatography where the stationary phase is supported on filter paper and the mobile phase moves through capillary action. Key aspects of paper chromatography covered include the principle, factors affecting separation, and different techniques such as ascending and descending development.
HPTLC

This document discusses high performance thin layer chromatography (HPTLC). It begins with an introduction and overview of HPTLC principles, instrumentation, differences from TLC, and application steps. The document then provides more details on the HPTLC instrumentation components, factors affecting separation, common stationary and mobile phases used, and application techniques. It concludes by discussing detection methods and some key applications of HPTLC in pharmaceuticals, food analysis, clinical studies, and forensics.
Theory of high performance liquid chromatography ppt

This document provides an overview of the theory of high performance liquid chromatography (HPLC). It discusses key concepts such as the retention factor (k), which is a measure of how long a compound is retained on the column. Selectivity (α) refers to the ability to distinguish between sample components, and is calculated as a ratio of the k values. Resolution (Rs) is the most important measure of separation, and depends on factors like k, α, and the number of theoretical plates (N). N is a measure of column efficiency, and the height equivalent of a theoretical plate (HETP) describes efficiency. The document outlines how these parameters can be optimized to improve separation and resolution.
What's hot (20)
Silica Gel | Aluminium Oxide Column chroamtography

Silica Gel | Aluminium Oxide Column chroamtography
Theory of high performance liquid chromatography ppt

Theory of high performance liquid chromatography ppt
Similar to Column Chromatography
Applied Pharmacognosy | Chromatography

Principles of chromatography
Chromatography’ is an analytical technique commonly used for separating a mixture of chemical substances into its individual components, so that the individual components can be thoroughly analyzed. There are many types of chromatography e.g., liquid chromatography, gas chromatography, ion-exchange chromatography, affinity chromatography, but all of these employ the same basic principles.
Chromatography is a separation technique that every organic chemist and biochemist is familiar with. I, myself, being an organic chemist, have routinely carried out chromatographic separations of a variety of mixture of compounds in the lab. In fact, I was leafing through my research slides and came across a pictorial representation of an actual chromatographic separation that I had carried out in the lab
DSE-2, ANALYTICAL METHODS -Ch-II.pptx

The document discusses various analytical chromatography techniques. It describes chromatography as separating components through distribution between two immiscible phases, with one stationary and one mobile. The document outlines different types of chromatography including column chromatography, thin layer chromatography, gas chromatography, and ion exchange chromatography. It discusses the principles, techniques, and efficiency of these analytical methods.
Types of chromatography

Here are the key principles of the different chromatography techniques covered in the document:
- Paper chromatography relies on the differential migration rates of compounds through a stationary phase (filter paper) based on their varying interactions with the mobile phase solvent. Components separate based on differences in solubility and affinity for the mobile vs. stationary phases.
- Thin layer chromatography uses a thin stationary phase coating (e.g. silica gel) on a plate. Components separate based on differences in their partitioning behavior between the mobile liquid phase and stationary solid phase during capillary movement.
- Column chromatography uses a packed stationary phase within a column. Components separate based on differences in their distribution between the stationary phase adsorbent and percolating mobile phase
Colum chromatography

Introduction to chromatography, Definition of Chromatography, Types of column chromatography, Theory of chromatography, Practical considerations in column chromatography , Factors affecting efficiency of a column, Applications.
Separation methods

This document provides information about basic phytochemical screening. It discusses the aims of separating a given sample into its various components and calculating retardation values. It then reviews chromatography techniques, describing paper and thin layer chromatography that will be used in the experiment. The introduction defines separation and discusses different separation methods, focusing on chromatography. It classifies chromatographic processes and mechanisms, explaining surface adsorption, partition, ion exchange, and size exclusion chromatography.
Chromatography, types by different approaches, HPLC 

Chromatography was first developed in 1906 by Russian scientist Tswett who separated plant pigments using calcium carbonate columns. The term "chromatography" comes from the Greek words for "color" and "to write". Chromatography separates mixtures based on how their components interact and distribute between a stationary and mobile phase. High performance liquid chromatography (HPLC) uses high pressure to force a mobile phase through a column packed with tiny particles. HPLC provides efficient separation of mixtures and is commonly used in analytical and preparative applications.
ir spectroscopy principle introductuion procedure

Infrared spectroscopy is a technique used to identify functional groups in molecules by detecting the vibrational and rotational frequencies of chemical bonds. It works based on the absorption of infrared radiation by the molecule, which causes changes in the dipole moment of the bonds. There are two main types of vibrations detected - stretching and bending. Factors like coupled vibrations, hydrogen bonding and electronic effects influence the vibrational frequencies observed. IR spectroscopy has applications in structure elucidation, quantitative analysis, reaction monitoring and more.
Colloidal dispersions pdf

This document discusses colloidal dispersions and their properties. It defines colloidal dispersions as heterogeneous biphasic systems with dispersed particles in the nano size range of 1-1000 nm. Colloids can be classified as lyophilic, lyophobic, or association colloids based on particle-solvent interactions. Key optical properties of colloids include the Tyndall effect, light scattering measurements to determine particle size and molecular weight, and imaging with electron microscopes. Colloids also exhibit kinetic properties like Brownian motion, diffusion, osmotic pressure, and sedimentation rates related to particle size. Electrolytes can cause coagulation or precipitation of colloids according to the Schulze-
Columnchromatography

Column chromatography is a separation technique that uses a column packed with a stationary phase to separate mixtures based on how compounds partition between the stationary and mobile phases. Martin and Synge introduced partition column chromatography in 1941 using differences in how compounds partition between two liquid phases. Column chromatography can use a solid stationary phase for adsorption chromatography or a liquid stationary phase for partition chromatography. The technique works by selectively retaining compounds based on their interaction with and attraction to the stationary phase.
CHROMATOGRAPHY 

Chromatography is a technique used to separate and analyze the components in a complex mixture. The mixture is dissolved in a mobile phase that carries it through a column containing a stationary phase. The different components in the mixture travel through the column at different rates depending on their interaction with the stationary and mobile phases, allowing separation. The separated components can then be identified, purified, and quantified.
3. Chromatography SANJAY B.Sc.III.pptx

This document provides information about chromatography techniques. It begins with an overview of chromatography, noting that it is a separation technique based on differences in how compounds interact with mobile and stationary phases. It then discusses several milestones in the development of chromatography from its invention in 1903 to instrumental developments in the 1960s. The document outlines various chromatography methods including paper chromatography, column chromatography, thin layer chromatography, and gas chromatography. It provides details on the principles, methodology, applications and advantages/disadvantages of each technique. Overall, the document presents an introduction to key chromatography methods.
Pharmacognosy II notes

Separation and Isolation of plant constituents
Carbohydrates
Glycosides
Tannins
Volatile Oil
Resins & Resin combinations
ALKALOIDS
Lipids
Tumor inhibitors from plants
Pharmacognosy-complete (chromatography,carbohydrates,glycosides,lipids,Vos,ro...

Chromatography is a technique used to separate mixtures into individual components. It works by exploiting differences in how compounds partition between a stationary and mobile phase. There are two main types: adsorption chromatography uses a solid stationary phase, while partition chromatography uses an immobilized liquid stationary phase. Chromatography can be classified based on the stationary and mobile phases used. Factors like polarity and affinity influence how compounds separate. Chromatographic techniques like gas chromatography are useful for separating volatile mixtures.
New microsoft office power point presentation

Chromatography is a technique used to separate and quantify the components in a complex chemical mixture. The mixture is introduced into a column filled with small particles. Different compounds in the mixture have different affinities for the particles, resulting in compounds with weaker affinities moving down the column faster than those with stronger affinities. This separates the various compounds based on their relative affinities for the solid particles. The limit of detection is the lowest concentration likely to be reliably distinguished from the limit of blank, while the limit of quantitation is the lowest concentration that can be reliably detected and meets goals for bias and imprecision.
Chromatography

Chromatography is an analytical technique used to separate, identify, and quantify components in complex mixtures. It works based on the differential partitioning of molecules between a stationary and mobile phase. The document defines key terms related to chromatography like stationary phase, mobile phase, eluent, eluate, elution, chromatogram, and flow rate. It also describes different types of chromatography like liquid chromatography, gas chromatography, and high pressure liquid chromatography. The document provides details on different modes of chromatography like adsorption, partition, ion exchange, size exclusion, and affinity chromatography. It discusses enzyme purification steps and parameters for anion exchange and hydrophobic interaction chromatography columns. Key properties and suggested buffers for DEAE Sepharose Fast Flow column are also presented
Advanced techniques and laborotory equipments for biologists

Gas chromatography (GC) separates volatile compounds using an inert gas as the mobile phase. The sample is injected into a heated port to volatilize it. The gas mobile phase carries the volatilized sample through a heated column coated with a stationary phase that interacts with analytes. Components are separated based on differences in volatility and affinity for the stationary phase, then detected and recorded. GC is useful for separating volatile, non-polar compounds.
Chromatography

This document provides an overview of chromatography. It discusses the history and discovery of chromatography by Tswett in 1906. It then defines chromatography and describes the basic components of a chromatogram. The document classifies chromatography by mobile and stationary phase as well as by separation mechanism. It discusses various chromatography techniques including thin layer chromatography, column chromatography, gas chromatography, and high performance liquid chromatography. It also covers separation factors such as solute retention, capacity factor, and efficiency.
chromatography, HPLC

Chromatography is a physical separation technique that separates the components of a mixture based on their interactions with two phases - a stationary phase and a mobile phase. Mikhail Tswett discovered chromatography in 1906 while separating plant pigments. Chromatography has many applications and has contributed to 12 Nobel prizes between 1937-1972. There are several types of chromatography classified by the mobile phase (liquid, gas), stationary phase (thin layer, paper, column), or separation force (adsorption, partition, ion exchange, gel filtration, affinity). High performance liquid chromatography uses high pressure to allow for faster separation over minutes versus hours with other techniques. Chromatography techniques like thin layer chromatography, gas chromatography, and HPLC are used in various
Affinity chromatography 1

Ion exchange chromatography separates components based on their surface charge by using a stationary phase with oppositely charged functional groups. The document provides background on the history and development of ion exchange and other chromatography techniques. It explains the principles and applications of ion exchange chromatography, including how it uses resins and gradients to differentially elute ions based on their affinity for the stationary phase.
Colloids 

This document provides information about colloidal dispersions. It defines a colloid as a substance microscopically dispersed throughout another substance, with particle sizes between 1-1000nm. Colloids can be classified based on their physical state, nature of interactions, size, appearance, or electric charge. Key properties of colloids include Brownian motion, diffusion, sedimentation, viscosity, light scattering, and electrical behaviors like electrophoresis and electrosmosis. Colloids find applications in areas like therapy, absorption, solubility, stability, and drug targeting.
Similar to Column Chromatography (20)
Chromatography, types by different approaches, HPLC 

Chromatography, types by different approaches, HPLC
Pharmacognosy-complete (chromatography,carbohydrates,glycosides,lipids,Vos,ro...

Pharmacognosy-complete (chromatography,carbohydrates,glycosides,lipids,Vos,ro...
Advanced techniques and laborotory equipments for biologists

Advanced techniques and laborotory equipments for biologists
Recently uploaded
Applied Science: Thermodynamics, Laws & Methodology.pdf

When I was asked to give a companion lecture in support of ‘The Philosophy of Science’ (https://shorturl.at/4pUXz) I decided not to walk through the detail of the many methodologies in order of use. Instead, I chose to employ a long standing, and ongoing, scientific development as an exemplar. And so, I chose the ever evolving story of Thermodynamics as a scientific investigation at its best.
Conducted over a period of >200 years, Thermodynamics R&D, and application, benefitted from the highest levels of professionalism, collaboration, and technical thoroughness. New layers of application, methodology, and practice were made possible by the progressive advance of technology. In turn, this has seen measurement and modelling accuracy continually improved at a micro and macro level.
Perhaps most importantly, Thermodynamics rapidly became a primary tool in the advance of applied science/engineering/technology, spanning micro-tech, to aerospace and cosmology. I can think of no better a story to illustrate the breadth of scientific methodologies and applications at their best.
ESR spectroscopy in liquid food and beverages.pptx

With increasing population, people need to rely on packaged food stuffs. Packaging of food materials requires the preservation of food. There are various methods for the treatment of food to preserve them and irradiation treatment of food is one of them. It is the most common and the most harmless method for the food preservation as it does not alter the necessary micronutrients of food materials. Although irradiated food doesn’t cause any harm to the human health but still the quality assessment of food is required to provide consumers with necessary information about the food. ESR spectroscopy is the most sophisticated way to investigate the quality of the food and the free radicals induced during the processing of the food. ESR spin trapping technique is useful for the detection of highly unstable radicals in the food. The antioxidant capability of liquid food and beverages in mainly performed by spin trapping technique.
Shallowest Oil Discovery of Turkiye.pptx

The Petroleum System of the Çukurova Field - the Shallowest Oil Discovery of Türkiye, Adana
Equivariant neural networks and representation theory

Or: Beyond linear.
Abstract: Equivariant neural networks are neural networks that incorporate symmetries. The nonlinear activation functions in these networks result in interesting nonlinear equivariant maps between simple representations, and motivate the key player of this talk: piecewise linear representation theory.
Disclaimer: No one is perfect, so please mind that there might be mistakes and typos.
dtubbenhauer@gmail.com
Corrected slides: dtubbenhauer.com/talks.html
BREEDING METHODS FOR DISEASE RESISTANCE.pptx

Plant breeding for disease resistance is a strategy to reduce crop losses caused by disease. Plants have an innate immune system that allows them to recognize pathogens and provide resistance. However, breeding for long-lasting resistance often involves combining multiple resistance genes
如何办理(uvic毕业证书)维多利亚大学毕业证本科学位证书原版一模一样

原版纸张【微信:741003700 】【(uvic毕业证书)维多利亚大学毕业证】【微信:741003700 】学位证,留信认证(真实可查,永久存档)offer、雅思、外壳等材料/诚信可靠,可直接看成品样本,帮您解决无法毕业带来的各种难题!外壳,原版制作,诚信可靠,可直接看成品样本。行业标杆!精益求精,诚心合作,真诚制作!多年品质 ,按需精细制作,24小时接单,全套进口原装设备。十五年致力于帮助留学生解决难题,包您满意。
本公司拥有海外各大学样板无数,能完美还原海外各大学 Bachelor Diploma degree, Master Degree Diploma
1:1完美还原海外各大学毕业材料上的工艺:水印,阴影底纹,钢印LOGO烫金烫银,LOGO烫金烫银复合重叠。文字图案浮雕、激光镭射、紫外荧光、温感、复印防伪等防伪工艺。材料咨询办理、认证咨询办理请加学历顾问Q/微741003700
留信网认证的作用:
1:该专业认证可证明留学生真实身份
2:同时对留学生所学专业登记给予评定
3:国家专业人才认证中心颁发入库证书
4:这个认证书并且可以归档倒地方
5:凡事获得留信网入网的信息将会逐步更新到个人身份内,将在公安局网内查询个人身份证信息后,同步读取人才网入库信息
6:个人职称评审加20分
7:个人信誉贷款加10分
8:在国家人才网主办的国家网络招聘大会中纳入资料,供国家高端企业选择人才
Thornton ESPP slides UK WW Network 4_6_24.pdf

ESPP presentation to EU Waste Water Network, 4th June 2024 “EU policies driving nutrient removal and recycling
and the revised UWWTD (Urban Waste Water Treatment Directive)”
bordetella pertussis.................................ppt

Bordettela is a gram negative cocobacilli spread by air born drop let
SAR of Medicinal Chemistry 1st by dk.pdf

In this presentation include the prototype drug SAR on thus or with their examples .
Syllabus of Second Year B. Pharmacy
2019 PATTERN.
Phenomics assisted breeding in crop improvement

As the population is increasing and will reach about 9 billion upto 2050. Also due to climate change, it is difficult to meet the food requirement of such a large population. Facing the challenges presented by resource shortages, climate
change, and increasing global population, crop yield and quality need to be improved in a sustainable way over the coming decades. Genetic improvement by breeding is the best way to increase crop productivity. With the rapid progression of functional
genomics, an increasing number of crop genomes have been sequenced and dozens of genes influencing key agronomic traits have been identified. However, current genome sequence information has not been adequately exploited for understanding
the complex characteristics of multiple gene, owing to a lack of crop phenotypic data. Efficient, automatic, and accurate technologies and platforms that can capture phenotypic data that can
be linked to genomics information for crop improvement at all growth stages have become as important as genotyping. Thus,
high-throughput phenotyping has become the major bottleneck restricting crop breeding. Plant phenomics has been defined as the high-throughput, accurate acquisition and analysis of multi-dimensional phenotypes
during crop growing stages at the organism level, including the cell, tissue, organ, individual plant, plot, and field levels. With the rapid development of novel sensors, imaging technology,
and analysis methods, numerous infrastructure platforms have been developed for phenotyping.
原版制作(carleton毕业证书)卡尔顿大学毕业证硕士文凭原版一模一样

原版纸张【微信:741003700 】【(carleton毕业证书)卡尔顿大学毕业证】【微信:741003700 】学位证,留信认证(真实可查,永久存档)offer、雅思、外壳等材料/诚信可靠,可直接看成品样本,帮您解决无法毕业带来的各种难题!外壳,原版制作,诚信可靠,可直接看成品样本。行业标杆!精益求精,诚心合作,真诚制作!多年品质 ,按需精细制作,24小时接单,全套进口原装设备。十五年致力于帮助留学生解决难题,包您满意。
本公司拥有海外各大学样板无数,能完美还原海外各大学 Bachelor Diploma degree, Master Degree Diploma
1:1完美还原海外各大学毕业材料上的工艺:水印,阴影底纹,钢印LOGO烫金烫银,LOGO烫金烫银复合重叠。文字图案浮雕、激光镭射、紫外荧光、温感、复印防伪等防伪工艺。材料咨询办理、认证咨询办理请加学历顾问Q/微741003700
留信网认证的作用:
1:该专业认证可证明留学生真实身份
2:同时对留学生所学专业登记给予评定
3:国家专业人才认证中心颁发入库证书
4:这个认证书并且可以归档倒地方
5:凡事获得留信网入网的信息将会逐步更新到个人身份内,将在公安局网内查询个人身份证信息后,同步读取人才网入库信息
6:个人职称评审加20分
7:个人信誉贷款加10分
8:在国家人才网主办的国家网络招聘大会中纳入资料,供国家高端企业选择人才
Travis Hills' Endeavors in Minnesota: Fostering Environmental and Economic Pr...

Travis Hills of Minnesota developed a method to convert waste into high-value dry fertilizer, significantly enriching soil quality. By providing farmers with a valuable resource derived from waste, Travis Hills helps enhance farm profitability while promoting environmental stewardship. Travis Hills' sustainable practices lead to cost savings and increased revenue for farmers by improving resource efficiency and reducing waste.
Micronuclei test.M.sc.zoology.fisheries.

Current Ms word generated power point presentation covers major details about the micronuclei test. It's significance and assays to conduct it. It is used to detect the micronuclei formation inside the cells of nearly every multicellular organism. It's formation takes place during chromosomal sepration at metaphase.
The use of Nauplii and metanauplii artemia in aquaculture (brine shrimp).pptx

Although Artemia has been known to man for centuries, its use as a food for the culture of larval organisms apparently began only in the 1930s, when several investigators found that it made an excellent food for newly hatched fish larvae (Litvinenko et al., 2023). As aquaculture developed in the 1960s and ‘70s, the use of Artemia also became more widespread, due both to its convenience and to its nutritional value for larval organisms (Arenas-Pardo et al., 2024). The fact that Artemia dormant cysts can be stored for long periods in cans, and then used as an off-the-shelf food requiring only 24 h of incubation makes them the most convenient, least labor-intensive, live food available for aquaculture (Sorgeloos & Roubach, 2021). The nutritional value of Artemia, especially for marine organisms, is not constant, but varies both geographically and temporally. During the last decade, however, both the causes of Artemia nutritional variability and methods to improve poorquality Artemia have been identified (Loufi et al., 2024).
Brine shrimp (Artemia spp.) are used in marine aquaculture worldwide. Annually, more than 2,000 metric tons of dry cysts are used for cultivation of fish, crustacean, and shellfish larva. Brine shrimp are important to aquaculture because newly hatched brine shrimp nauplii (larvae) provide a food source for many fish fry (Mozanzadeh et al., 2021). Culture and harvesting of brine shrimp eggs represents another aspect of the aquaculture industry. Nauplii and metanauplii of Artemia, commonly known as brine shrimp, play a crucial role in aquaculture due to their nutritional value and suitability as live feed for many aquatic species, particularly in larval stages (Sorgeloos & Roubach, 2021).
3D Hybrid PIC simulation of the plasma expansion (ISSS-14)

3D Particle-In-Cell (PIC) algorithm,
Plasma expansion in the dipole magnetic field.
Comparing Evolved Extractive Text Summary Scores of Bidirectional Encoder Rep...

Comparing Evolved Extractive Text Summary Scores of Bidirectional Encoder Rep...University of Maribor
Slides from:
11th International Conference on Electrical, Electronics and Computer Engineering (IcETRAN), Niš, 3-6 June 2024
Track: Artificial Intelligence
https://www.etran.rs/2024/en/home-english/Recently uploaded (20)
Applied Science: Thermodynamics, Laws & Methodology.pdf

Applied Science: Thermodynamics, Laws & Methodology.pdf
ESR spectroscopy in liquid food and beverages.pptx

ESR spectroscopy in liquid food and beverages.pptx
Equivariant neural networks and representation theory

Equivariant neural networks and representation theory
bordetella pertussis.................................ppt

bordetella pertussis.................................ppt
Travis Hills' Endeavors in Minnesota: Fostering Environmental and Economic Pr...

Travis Hills' Endeavors in Minnesota: Fostering Environmental and Economic Pr...
The use of Nauplii and metanauplii artemia in aquaculture (brine shrimp).pptx

The use of Nauplii and metanauplii artemia in aquaculture (brine shrimp).pptx
3D Hybrid PIC simulation of the plasma expansion (ISSS-14)

3D Hybrid PIC simulation of the plasma expansion (ISSS-14)
Comparing Evolved Extractive Text Summary Scores of Bidirectional Encoder Rep...

Comparing Evolved Extractive Text Summary Scores of Bidirectional Encoder Rep...
Column Chromatography
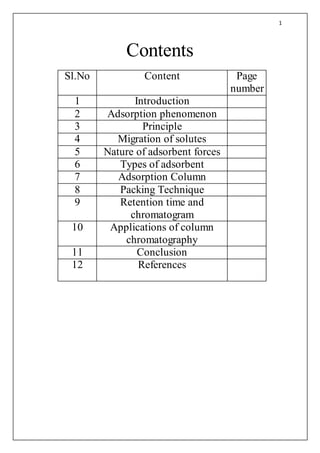
- 1. 1 Contents Sl.No Content Page number 1 Introduction 2 Adsorption phenomenon 3 Principle 4 Migration of solutes 5 Nature of adsorbent forces 6 Types of adsorbent 7 Adsorption Column 8 Packing Technique 9 Retention time and chromatogram 10 Applications of column chromatography 11 Conclusion 12 References
- 2. 2 COLUMN CHROMATOGRAPHY OBJECTIVE The aim of this assignment is to know how to separate two substances using column chromatography. As an example, methylene blue and methyl orange will be separated using an alumina packed column. The separated substances will then be analyzed spectrophotometrically using a visible spectrophotometer. INTRODUCTION Chromatography is a non destructive process for resolving a multicomponent mixture into its individual fractions.
- 3. 3 It was discovered by Dr.M.Tswett in 1906. We already came across paper chromatography, thin layer chromatography by using chromatography we can separate the mixture of amino acids, which resembles one another in chemical properties ,can be separated fairly and rapidly. There are different kinds of different types of chromatography which differ in the mobile phase and stationary phase. Column chromatography is type of chromatographic technique was developed by the American botanist D.T. Day in1900 and M. Tswett in 1906 used absorption columns in their investigations of plant pigments. Column chromatography is one of the useful methods for separation and purification of both solids and liquids. Column chromatography is also known as adsorption chromatography. In this chromatography is of solid- liquid technique in which the stationary phase is a solid and mobile phase is liquid. ADSORPTION PHENOMENON The column chromatography involves adsorption, partition and ion exchange phenomenon 1. Adsorption Column Chromatography Here in this type of chromatography the substances are absorbed by adsorbent packed in the column 2. Partition Column Chromatography Here in this type of chromatography the separation of components of a mixture distribute themselves in different ratios between two different solvents. The column is packed with silica gel or cellulose which contain some amount of water. 3. Ion-exchange Column Chromatography
- 4. 4 Here in this type of chromatography the usual solvent is water and selective desorption of ions is carried out by altering the pH or concentration of ions in the eluting solvent. PRINCIPLE The principle involved in the column chromatography is based on differential adsorption of substance by the usual adsorbents used in column chromatography are silica, alumina, calcium carbonate ,calcium phosphate, magnesia, starch etc. Selection of solvent is based on the nature of both the solvent and adsorbent. The rate at which the components of a mixture are separated depends on the adsorbent and polarity of a solvent. If the activity of the adsorbent is high and polarity of the solvent very low but gives a good separation, on the other hand, If the activity adsorbent is low and polarity of the solvent is high the separation is rapid but gives only poor separation i.e., the components separated are not 100% pure. Column chromatography involves a mobile phase flowing over a stationary phase. Migration Rates of Solutes The Partition Coefficient
- 5. 5 An analyte is in equilibrium between the two phases; Amobile ↔ Astationary MS CCK Where the equilibrium constant K is called the partition coefficient. CS: Molar concentration of analyte in stationary phase CM: Molar concentration of analyte in mobile phase The above diagram showing the separation of a mixture of components A and B by column chromatography NATURE OF ADSORBENT FORCES1 The adsorbent provides a very large surface area and has the ability to absorb chemical substances on its surface through some physical and chemical interactions and they are Van der Waals forces, inductive forces, hydrogen bonding, charge transfer and covalent bonding. i. Van der Waals forces Van der Waals forces hold neutral molecule together in the liquid or solid state. Adsorption based on this is purely physical in nature characterized by low adsorption energies and rapid equilibrium takes place and results in good separation .Adsorption of non-polar solutes on non polar adsorbents occurs by Van der Waals forces as, for example, in case of hydrocarbons and graphite.
- 6. 6 ii. Inductive forces Inductive forces or dipole-dipole attractions arise when a chemical bond has a permanent electrical field with it (e.g.,C-NO2 , C-Cl etc). The electrons of an adjacent atom or group or molecule get polarized under the influence of this field. This in turn gives rise to an induced dipole-dipole attraction between the adsorbent and solute. Many adsorption on alumina illustrate operation of these inductive forces. iii. Hydrogen bonding Hydrogen bonding becomes important when the solutes have a proton donor group which can undergo hydrogen bonding with the polar groups present at the surface of adsorbent (e.g., the surface hydroxyl groups possessed by silica or alumina). These surface hydroxyl groups will themselves act as proton-donor groups, thus giving rise to hydrogen bonding on coming in contact with, for example, ethers, nitriles or aromatic hydrocarbons. iv. Charge transfer The contribution of charge transfer to adsorption energy is reported to be very little in this case of most compounds. An adsorbed complex of the type, (Solute)+ (Adsorbent site) - results by the transfer of an electron from the solute to a surface site. v. Covalent bonding Covalent bonding results to the operation of relatively strong chemical forces between the solute and the adsorbent. Components of the mixture obtained by chromatographic separation may not possess high degree of purity in cases where these strong forces are operating.
- 7. 7 Types of adsorbents2 Adsorbent is generally an active solid with a large surface area. Weak adsorbents include talc, sucrose, starch, insulin etc. Intermediate adsorbents are slaked lime, magnesia, CaCO3, Ca3(PO4)2 , Na2SO4 etc Strong adsorbents are alumina, bauxite, charcoal etc. Highly active adsorbents may give rise to irreversible solute adsorption. Silica gel (acidic) may strongly retain basic compounds whereas alumina (basic) should not be used for base-sensitive compounds. Equilibrium is attained as the adsorbed layer consists of a monolayer covering the entire adsorbent volume (Va) given by, Va=3.5 ×10-8cm × surface area in cm2/ g-0.01% (H2O) SOLVENT SYSTEM2 As we know the separation in a column involves adsorption, partition and ion exchange phenomenon so the choice of solvent depends on these properties and also depends on polarity and solubility. For placing the solute on column, developing the chromatogram and eluting the adsorbed materials, different solvents will be used. Generally a single solvent is used but in certain cases simultaneous use of two or more solvents is better. The purity of the solvents is very important because impurities may slightly affect the column performance. The role of solvents is very important because mobile phase molecules compete with solute molecules for polar adsorption sites.
- 8. 8 The stronger the interaction between mobile phase and stationary phase, the weaker the solute adsorption. Solvent also elute the components of each separated zones. The classification of solvents according to their strength of adsorption is known as elutropic series. The eluting power of solvents is practically proportional to their dielectric constants. Eluting power of solvents2 Solvent ϵ293 Petroleum ether 1.90 Benzene 2.28 n-Propanol 21.80 Water 80.40 Chloroform 4.81 Carbon tetrachloride 2.24 Ethanol 25.80 Pyridine 12.40 Formamide 84.0 Absolute alcohol 4.34 Acetone 21.40 Methanol 33.60 Increasing order of polarity of common solvents Increasing order of polarity of common solvents is: Petroleum ether < carbon tetrachloride < Cyclohexane <Carbon disulphide <Ether <acetone <Benzene <Chloroform <Alcohols <Water <Pyridine <Organic acids. ADSORPTION COLUMN Adsorption column can be of any size, shape, length or design. Generally the size of the column is determined by the quantity of the mixture being
- 9. 9 fractioned. The geometry on the column depends on the form and size of the zones to be separated. The column is commonly made of pyrex glass. The smaller the diameter of the column, the more effective will be the separation and the bands will be more distinct. Chromatographic adsorption apparatus shown below. PACKING TECHNIQUES2 Packing of the column can be done in two ways 1) Wet packing For wet packing the column is clamped in a vertical position and thick slurry of the adsorbent in a suitable medium is poured through the open end. It is allowed to settle under gravity until a column of a desired height is obtained. The tap at the lower end is opened to allow the liquid to run out until it just covers the top medium. Wet
- 10. 10 packing is common with adsorbents like alumina and magnesia etc. 2) Dry packing In dry packing the dry powdered adsorbent is poured though the open end. Vacuum is created at the bottom and column is tapped with a light object until no more settling takes place. Ensure that the top is solid and unbroken. Retention Time (tR ) The time it takes after sample injection for the analyte peak to reach the detector is called retention time and its symbol is Rt . RtLv MtLu where v : average linear rate of analyte migration L: Length of column packing u : Average linear rate of movement of molecules of mobile phase Mt : Time required for an average, molecule of mobile phase to pass through the column, dead time. Chromatograms If a detector that responds to the presence of an analyte is placed at the end of the column and its signal is plotted as a function of time (volume of added mobile phase), a series of peaks is obtained. Such a plot, called a chromatogram, is useful for both qualitative and quantitative analysis.
- 11. 11 APPLICATIONS OF COLUMN CHROMATOGRAPHY The applications of column chromatography are: In the separation of mixtures into pure individual components. Removal impurities and in the purification of compounds. The the concentration of substance from dilute solutions such as those obtained when natural products are extracted with large volumes of solvents from the leaves, trees, roots and barks. Determination of homogeneity of chemical substances. Identification of unknown compounds. In the separation of geometrical isomers, diastereomers, recemates and tautomers.
- 12. 12 REFERENCES Dr.H.Kaur.”Instrumental methods of chemical analysis”, 9th Edition , Pragathi Prakashana Publication :1020-1026 R.P. BUDHIRAJA. Copy right 2OO4”Separation Chemistry”, New Age International (P) Ltd. :79-80