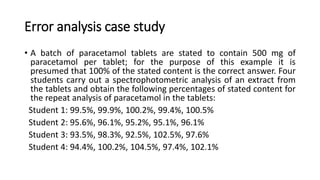
The document discusses instrumental analysis in analytical chemistry, emphasizing qualitative and quantitative methods, and identifying common sources of errors such as incorrect weighing and calibration issues. It outlines criteria for selecting analytical methods, focusing on parameters like precision, accuracy, specificity, sensitivity, and robustness while also detailing approaches to minimize errors. An example analysis of paracetamol tablets by different students illustrates variations in precision and accuracy among their results.