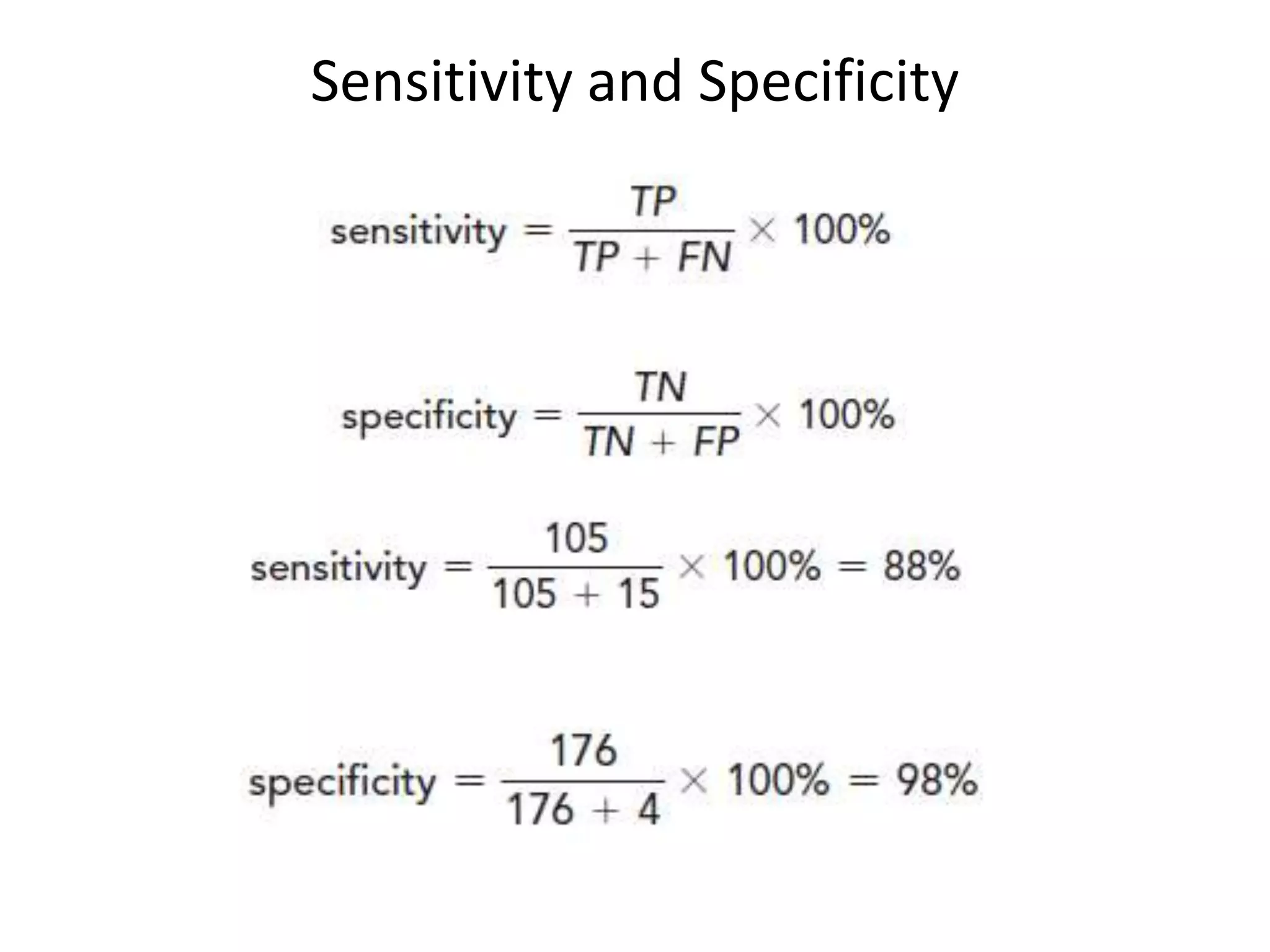
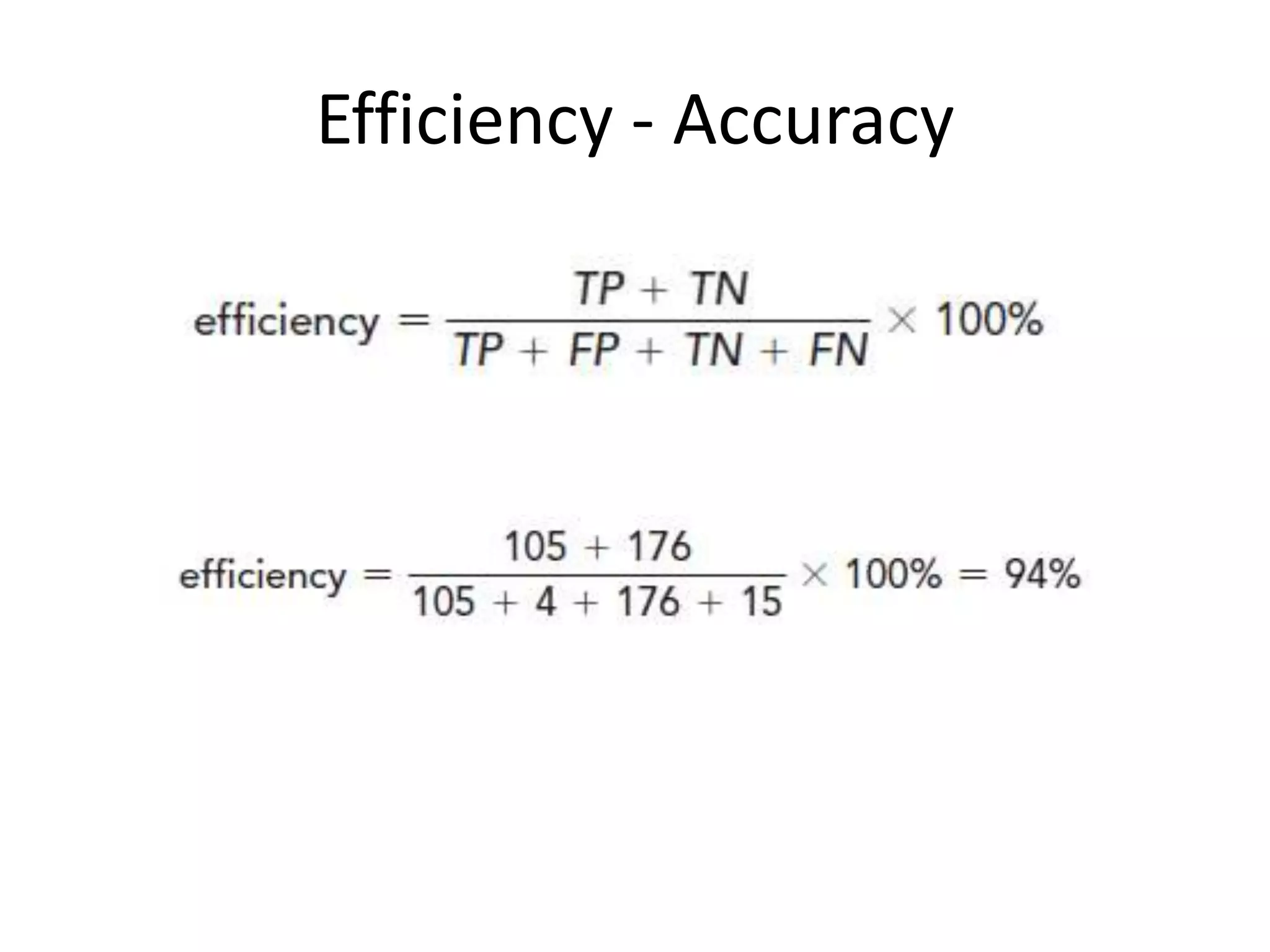
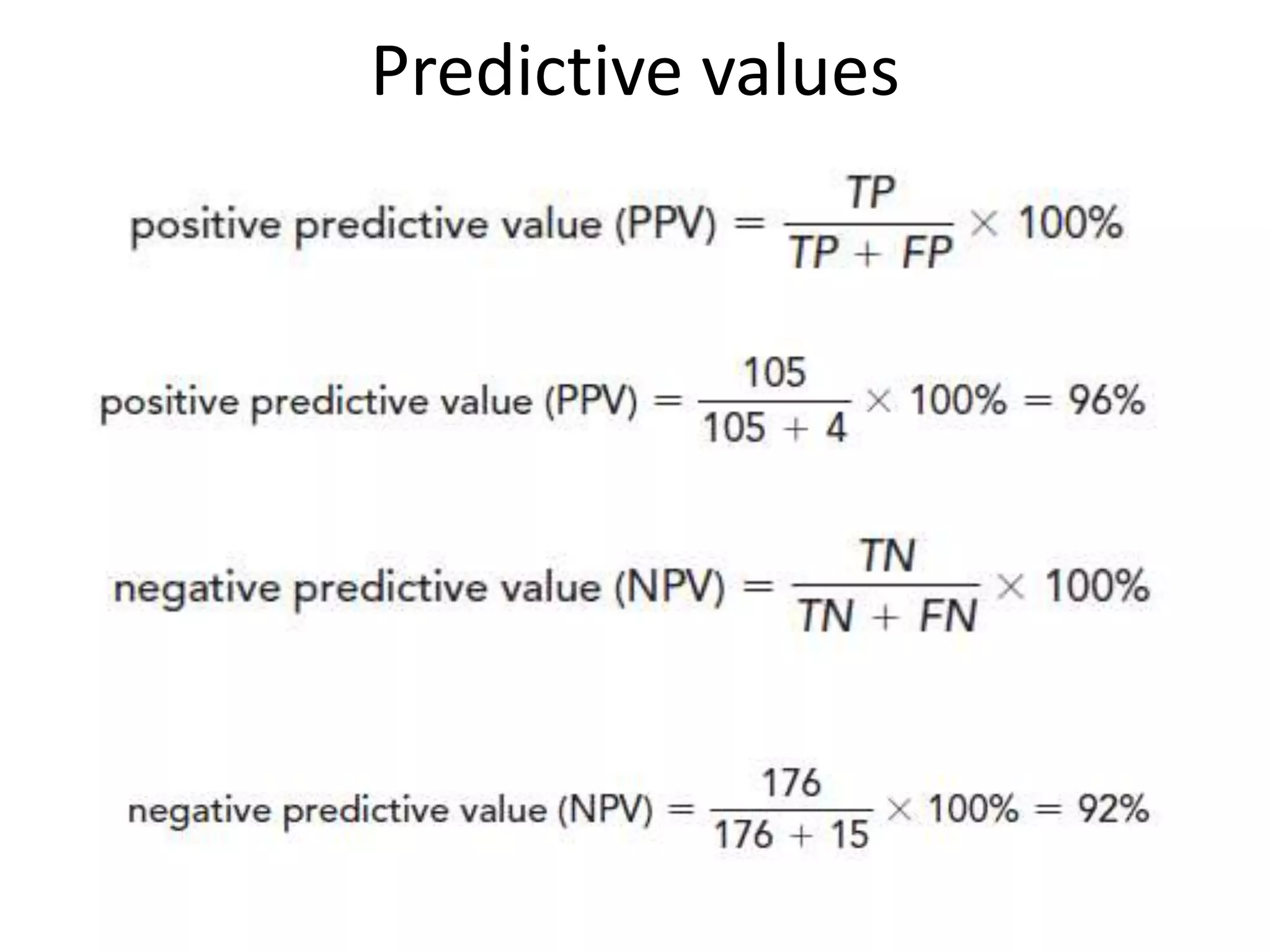
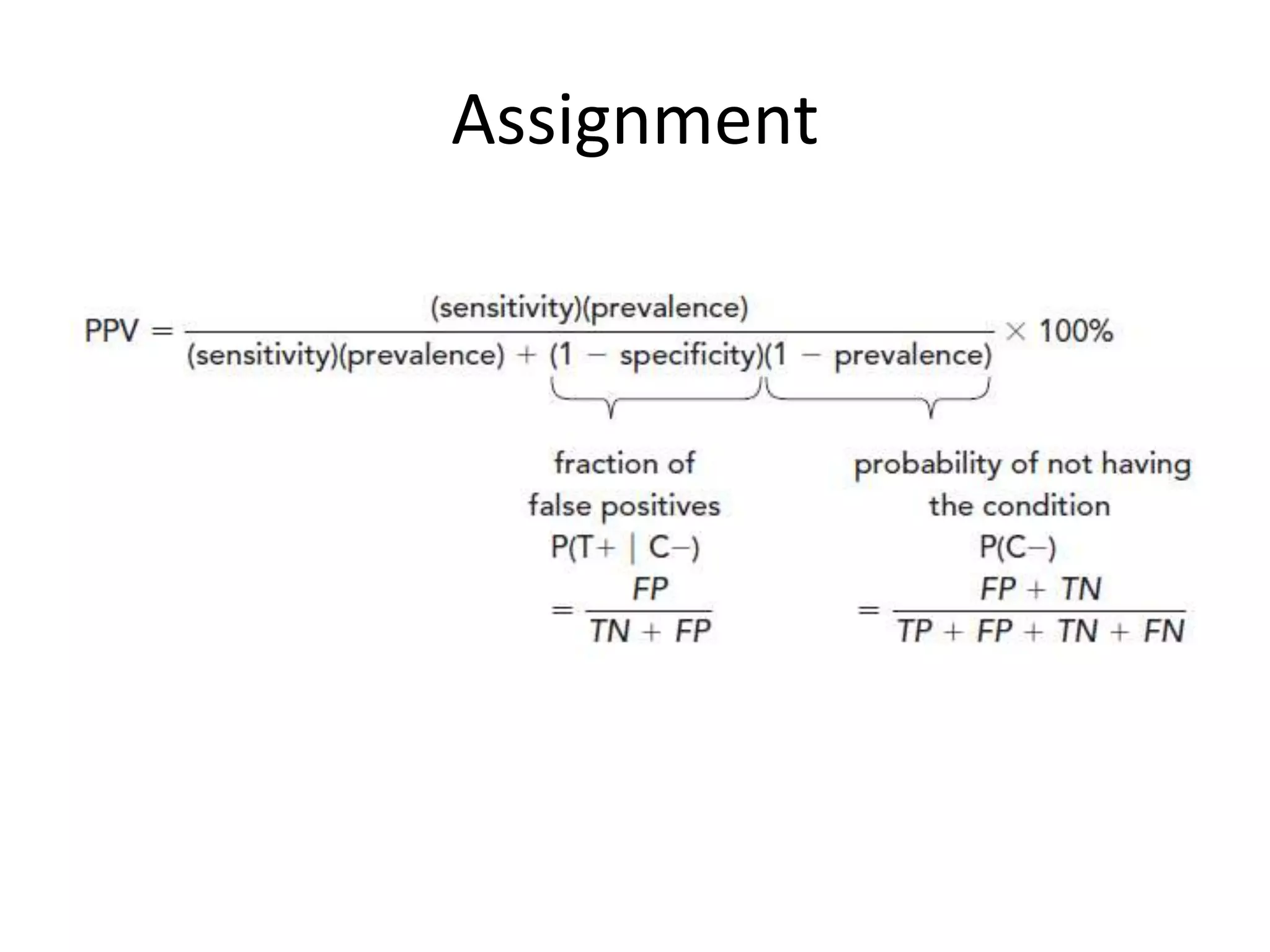
Here are the steps to calculate sensitivity, specificity, positive predictive value, negative predictive value, and efficiency for the given diagnostic test:
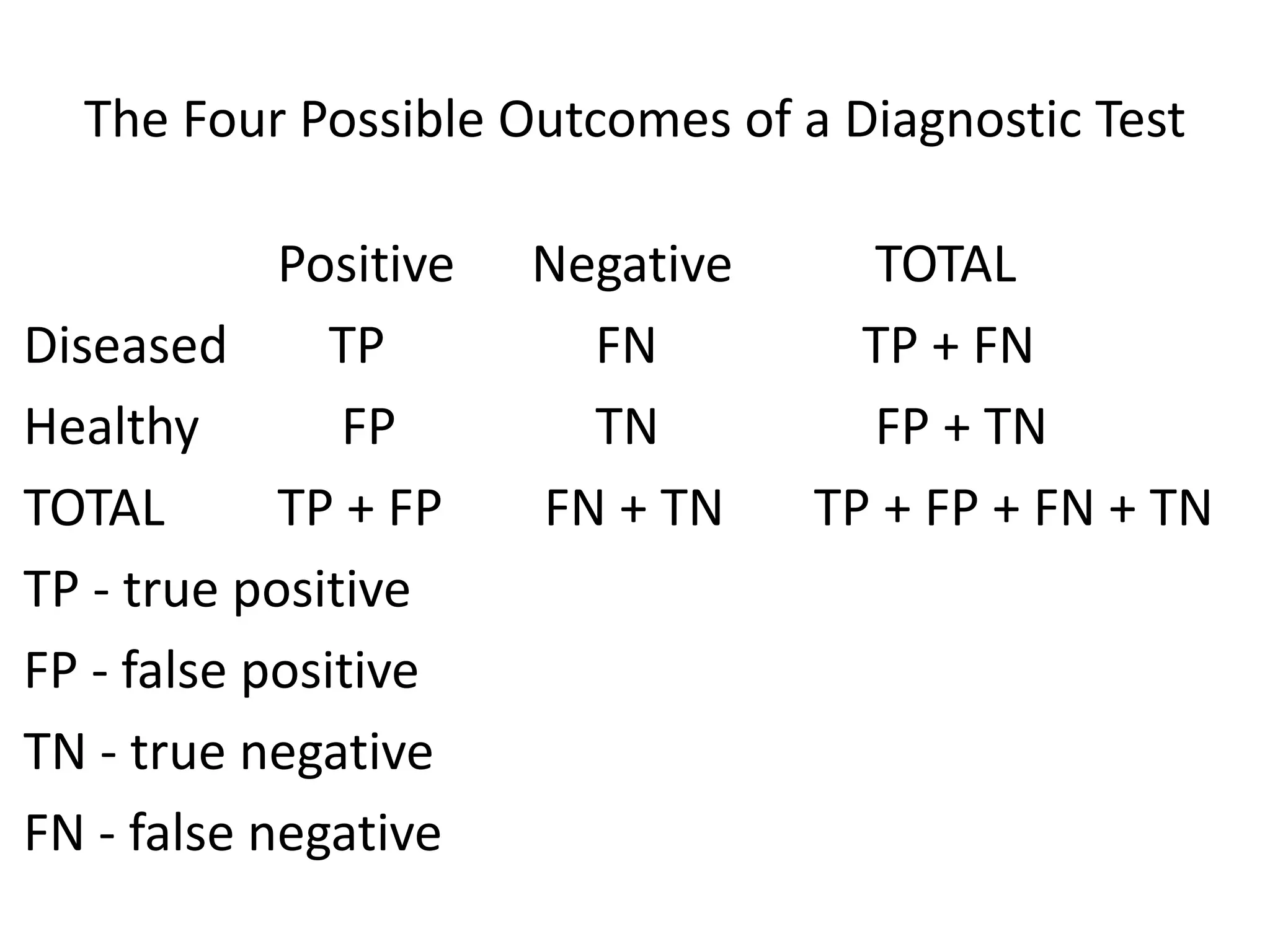
* True Positives (TP) = Number of HIV+ samples correctly identified as positive by the test = 120 - 15 = 105
* True Negatives (TN) = Number of HIV- samples correctly identified as negative by the test = 300 - (120 + 4) = 176
* False Positives (FP) = Number of HIV- samples incorrectly identified as positive by the test = 4
* False Negatives (FN) = Number of HIV+ samples incorrectly identified as negative by the test = 15
Sensitivity = TP / (TP + FN) = 105 / (105