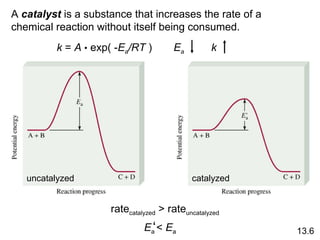
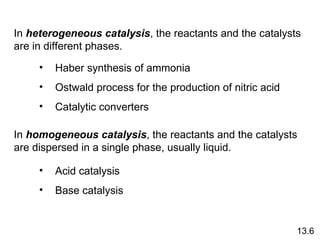
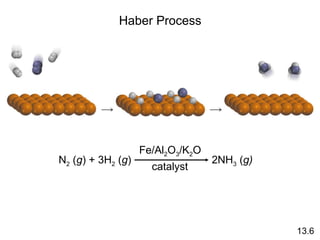
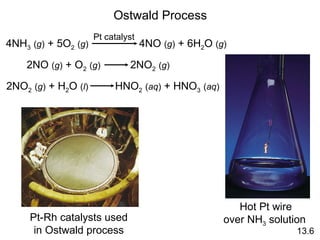
The document discusses chemical kinetics and reaction rates. It covers zero-order, first-order and second-order reactions, reaction mechanisms, and catalyzed reactions. Key concepts include determining the rate law from experimental data, the relationship between reaction order and the rate law, and how catalysts lower the activation energy and increase reaction rates without being consumed in the reaction.

![Chemical Kinetics Thermodynamics – does a reaction take place? Kinetics – how fast does a reaction proceed? Reaction rate is the change in the concentration of a reactant or a product with time ( M /s). [A] = change in concentration of A over time period t [B] = change in concentration of B over time period t Because [A] decreases with time, [A] is negative . 13.1 A B rate = - [A] t rate = [B] t](https://image.slidesharecdn.com/chemicalkineticsonline-110303140435-phpapp02/85/Chemical-kinetics-online_-2-320.jpg)
![13.1 A B rate = - [A] t rate = [ B ] t time](https://image.slidesharecdn.com/chemicalkineticsonline-110303140435-phpapp02/85/Chemical-kinetics-online_-3-320.jpg)
![ [Br 2 ] Absorption 13.1 Br 2 ( aq ) + HCOOH ( aq ) 2Br - ( aq ) + 2H + ( aq ) + CO 2 ( g ) time 393 nm light Detector 393 nm Br 2 ( aq )](https://image.slidesharecdn.com/chemicalkineticsonline-110303140435-phpapp02/85/Chemical-kinetics-online_-4-320.jpg)
![instantaneous rate = rate for specific instance in time 13.1 Br 2 ( aq ) + HCOOH ( aq ) 2Br - ( aq ) + 2H + ( aq ) + CO 2 ( g ) average rate = - [Br 2 ] t = - [Br 2 ] final – [Br 2 ] initial t final - t initial slope of tangent slope of tangent slope of tangent](https://image.slidesharecdn.com/chemicalkineticsonline-110303140435-phpapp02/85/Chemical-kinetics-online_-5-320.jpg)
![rate [Br 2 ] rate = k [Br 2 ] 13.1 = rate constant = 3.50 x 10 -3 s -1 k = rate [Br 2 ]](https://image.slidesharecdn.com/chemicalkineticsonline-110303140435-phpapp02/85/Chemical-kinetics-online_-6-320.jpg)
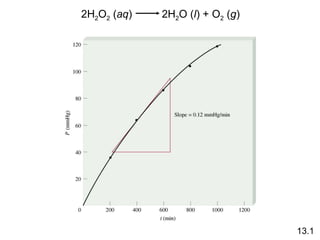
![PV = nRT 13.1 2H 2 O 2 ( aq ) 2H 2 O ( l ) + O 2 ( g ) P = RT = [O 2 ] RT n V [O 2 ] = P RT 1 rate = [O 2 ] t RT 1 P t = measure P over time](https://image.slidesharecdn.com/chemicalkineticsonline-110303140435-phpapp02/85/Chemical-kinetics-online_-7-320.jpg)
![Reaction Rates and Stoichiometry 13.1 Two moles of A disappear for each mole of B that is formed. 2A B rate = [B] t rate = - [A] t 1 2 a A + b B c C + d D rate = - [A] t 1 a = - [B] t 1 b = [C] t 1 c = [D] t 1 d](https://image.slidesharecdn.com/chemicalkineticsonline-110303140435-phpapp02/85/Chemical-kinetics-online_-9-320.jpg)
![13.1 Write the rate expression for the following reaction: CH 4 ( g ) + 2O 2 ( g ) CO 2 ( g ) + 2H 2 O ( g ) rate = - [CH 4 ] t = - [O 2 ] t 1 2 = [H 2 O] t 1 2 = [CO 2 ] t](https://image.slidesharecdn.com/chemicalkineticsonline-110303140435-phpapp02/85/Chemical-kinetics-online_-10-320.jpg)
![The Rate Law 13.2 The rate law expresses the relationship of the rate of a reaction to the rate constant and the concentrations of the reactants raised to some powers. Rate = k [A] x [B] y reaction is x th order in A reaction is y th order in B reaction is (x +y)th order overall a A + b B c C + d D](https://image.slidesharecdn.com/chemicalkineticsonline-110303140435-phpapp02/85/Chemical-kinetics-online_-11-320.jpg)
![rate = k [F 2 ] x [ClO 2 ] y Double [F 2 ] with [ClO 2 ] constant Rate doubles x = 1 Quadruple [ClO 2 ] with [F 2 ] constant Rate quadruples y = 1 rate = k [F 2 ][ClO 2 ] 13.2 F 2 ( g ) + 2ClO 2 ( g ) 2FClO 2 ( g )](https://image.slidesharecdn.com/chemicalkineticsonline-110303140435-phpapp02/85/Chemical-kinetics-online_-12-320.jpg)
![rate = k [F 2 ][ClO 2 ] Rate Laws Rate laws are always determined experimentally. Reaction order is always defined in terms of reactant (not product) concentrations. The order of a reactant is not related to the stoichiometric coefficient of the reactant in the balanced chemical equation. 13.2 F 2 ( g ) + 2ClO 2 ( g ) 2FClO 2 ( g ) 1](https://image.slidesharecdn.com/chemicalkineticsonline-110303140435-phpapp02/85/Chemical-kinetics-online_-13-320.jpg)
![rate = k [S 2 O 8 2- ] x [I - ] y Double [I - ], rate doubles (experiment 1 & 2) y = 1 Double [S 2 O 8 2- ], rate doubles (experiment 2 & 3) x = 1 = 0.08/ M • s 13.2 rate = k [S 2 O 8 2- ][I - ] Determine the rate law and calculate the rate constant for the following reaction from the following data: S 2 O 8 2- ( aq ) + 3I - ( aq ) 2SO 4 2- ( aq ) + I 3 - ( aq ) Experiment [S 2 O 8 2- ] [I - ] Initial Rate ( M /s) 1 0.08 0.034 2.2 x 10 -4 2 0.08 0.017 1.1 x 10 -4 3 0.16 0.017 2.2 x 10 -4 k = rate [S 2 O 8 2- ][I - ] = 2.2 x 10 -4 M /s (0.08 M )(0.034 M )](https://image.slidesharecdn.com/chemicalkineticsonline-110303140435-phpapp02/85/Chemical-kinetics-online_-14-320.jpg)
![First-Order Reactions 13.3 rate = k [A] k = = 1/s or s -1 [A] is the concentration of A at any time t [A] 0 is the concentration of A at time t =0 A product rate = - [A] t rate [A] M / s M = [A] t = k [A] - [A] = [A] 0 exp( -kt ) ln[A] = ln[A] 0 - kt](https://image.slidesharecdn.com/chemicalkineticsonline-110303140435-phpapp02/85/Chemical-kinetics-online_-15-320.jpg)
![ln[A] = ln[A] 0 - kt kt = ln[A] 0 – ln[A] t = = 66 s [A] 0 = 0.88 M [A] = 0.14 M 13.3 The reaction 2A B is first order in A with a rate constant of 2.8 x 10 -2 s -1 at 80 0 C. How long will it take for A to decrease from 0.88 M to 0.14 M ? ln[A] 0 – ln[A] k ln [A] 0 [A] k = ln 0.88 M 0.14 M 2.8 x 10 -2 s -1 =](https://image.slidesharecdn.com/chemicalkineticsonline-110303140435-phpapp02/85/Chemical-kinetics-online_-16-320.jpg)
![First-Order Reactions 13.3 The half-life , t ½ , is the time required for the concentration of a reactant to decrease to half of its initial concentration. t ½ = t when [A] = [A] 0 /2 = 1200 s = 20 minutes How do you know decomposition is first order? units of k (s -1 ) ln [A] 0 [A] 0 /2 k = t ½ ln2 k = 0.693 k = What is the half-life of N 2 O 5 if it decomposes with a rate constant of 5.7 x 10 -4 s -1 ? t ½ ln2 k = 0.693 5.7 x 10 -4 s -1 =](https://image.slidesharecdn.com/chemicalkineticsonline-110303140435-phpapp02/85/Chemical-kinetics-online_-17-320.jpg)
![First-order reaction 1 2 3 4 2 4 8 16 13.3 A product # of half-lives [A] = [A] 0 / n](https://image.slidesharecdn.com/chemicalkineticsonline-110303140435-phpapp02/85/Chemical-kinetics-online_-18-320.jpg)
![Second-Order Reactions 13.3 rate = k [A] 2 k = = 1/ M • s [A] is the concentration of A at any time t [A] 0 is the concentration of A at time t =0 t ½ = t when [A] = [A] 0 /2 A product rate = - [A] t rate [A] 2 M / s M 2 = [A] t = k [A] 2 - 1 [A] = 1 [A] 0 + kt t ½ = 1 k [A] 0](https://image.slidesharecdn.com/chemicalkineticsonline-110303140435-phpapp02/85/Chemical-kinetics-online_-19-320.jpg)
![Zero-Order Reactions 13.3 rate = k [A] 0 = k k = = M /s [A] is the concentration of A at any time t [A] 0 is the concentration of A at time t =0 t ½ = t when [A] = [A] 0 /2 [A] = [A] 0 - kt A product rate = - [A] t rate [A] 0 [A] t = k - t ½ = [A] 0 2 k](https://image.slidesharecdn.com/chemicalkineticsonline-110303140435-phpapp02/85/Chemical-kinetics-online_-20-320.jpg)
![Summary of the Kinetics of Zero-Order, First-Order and Second-Order Reactions 0 1 2 rate = k rate = k [A] rate = k [A] 2 ln[A] = ln[A] 0 - kt [A] = [A] 0 - kt 13.3 Order Rate Law Concentration-Time Equation Half-Life 1 [A] = 1 [A] 0 + kt t ½ ln2 k = t ½ = [A] 0 2 k t ½ = 1 k [A] 0](https://image.slidesharecdn.com/chemicalkineticsonline-110303140435-phpapp02/85/Chemical-kinetics-online_-21-320.jpg)
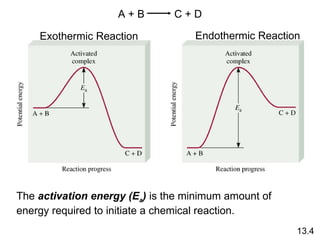


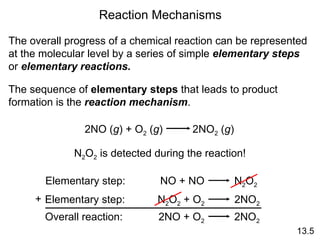
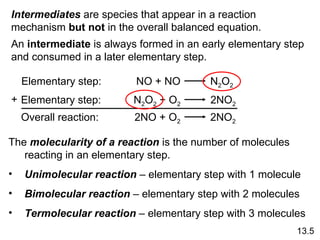
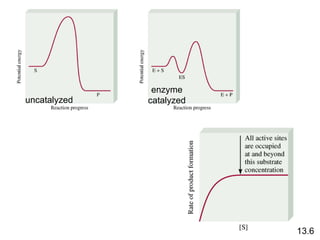
![rate = k [A] rate = k [A][B] rate = k [A] 2 Rate Laws and Elementary Steps 13.5 Writing plausible reaction mechanisms: The sum of the elementary steps must give the overall balanced equation for the reaction. The rate-determining step should predict the same rate law that is determined experimentally. Unimolecular reaction A products Bimolecular reaction A + B products Bimolecular reaction A + A products The rate-determining step is the slowest step in the sequence of steps leading to product formation.](https://image.slidesharecdn.com/chemicalkineticsonline-110303140435-phpapp02/85/Chemical-kinetics-online_-27-320.jpg)
![What is the equation for the overall reaction? What is the intermediate? NO 3 What can you say about the relative rates of steps 1 and 2? rate = k [NO 2 ] 2 is the rate law for step 1 so step 1 must be slower than step 2 13.5 The experimental rate law for the reaction between NO 2 and CO to produce NO and CO 2 is rate = k [NO 2 ] 2 . The reaction is believed to occur via two steps: Step 1: NO 2 + NO 2 NO + NO 3 Step 2: NO 3 + CO NO 2 + CO 2 NO 2 + CO NO + CO 2](https://image.slidesharecdn.com/chemicalkineticsonline-110303140435-phpapp02/85/Chemical-kinetics-online_-28-320.jpg)