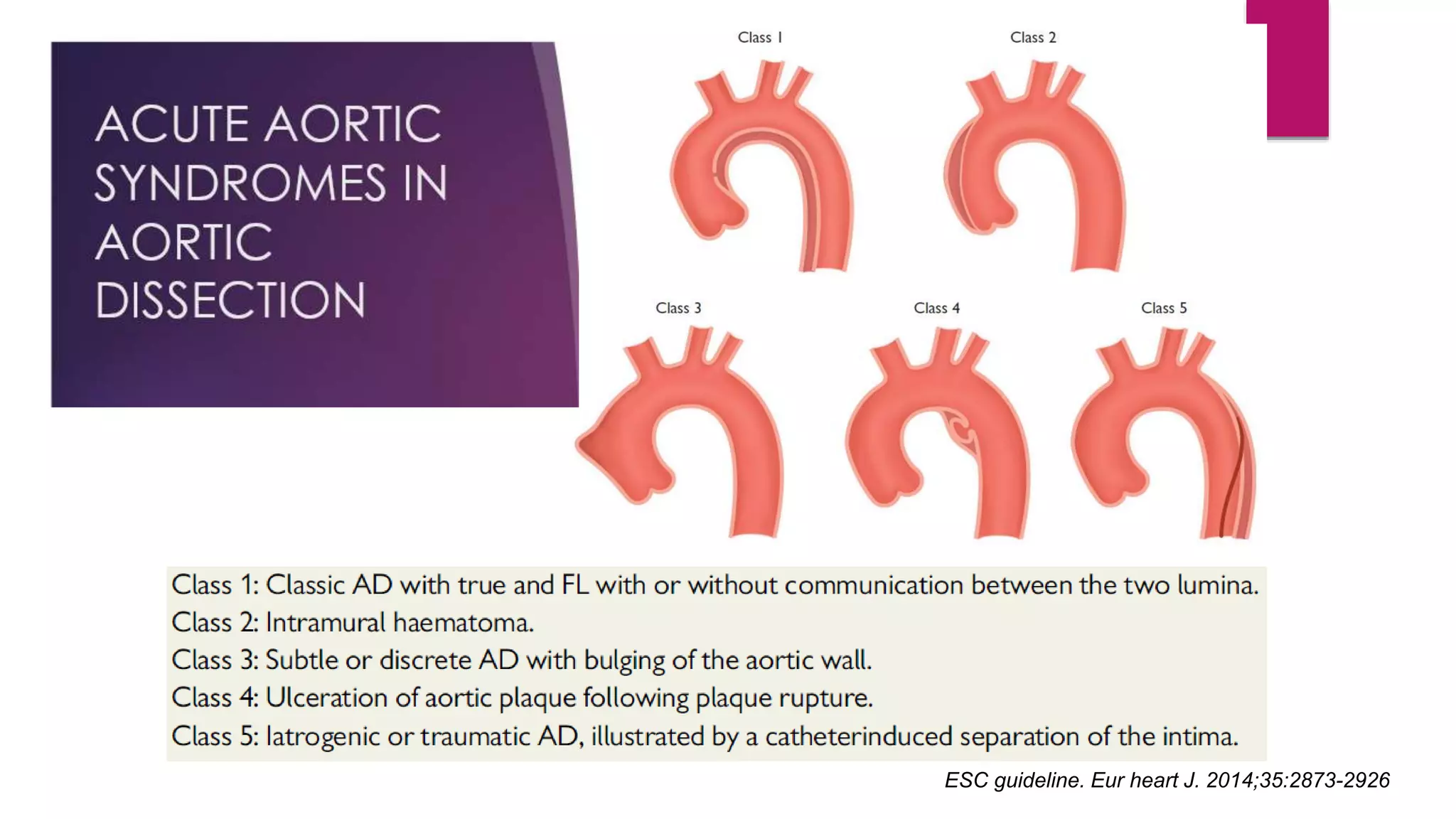
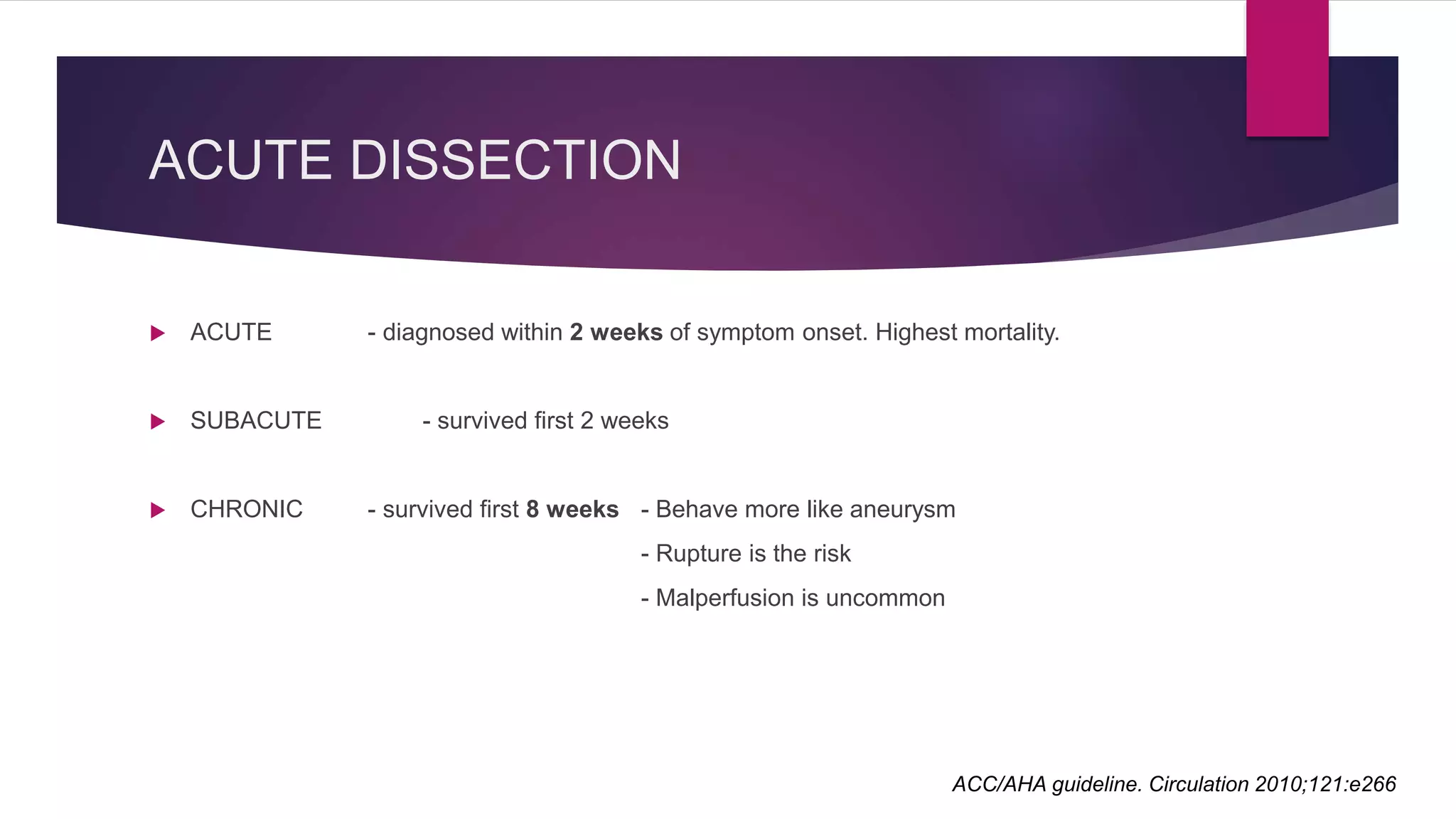
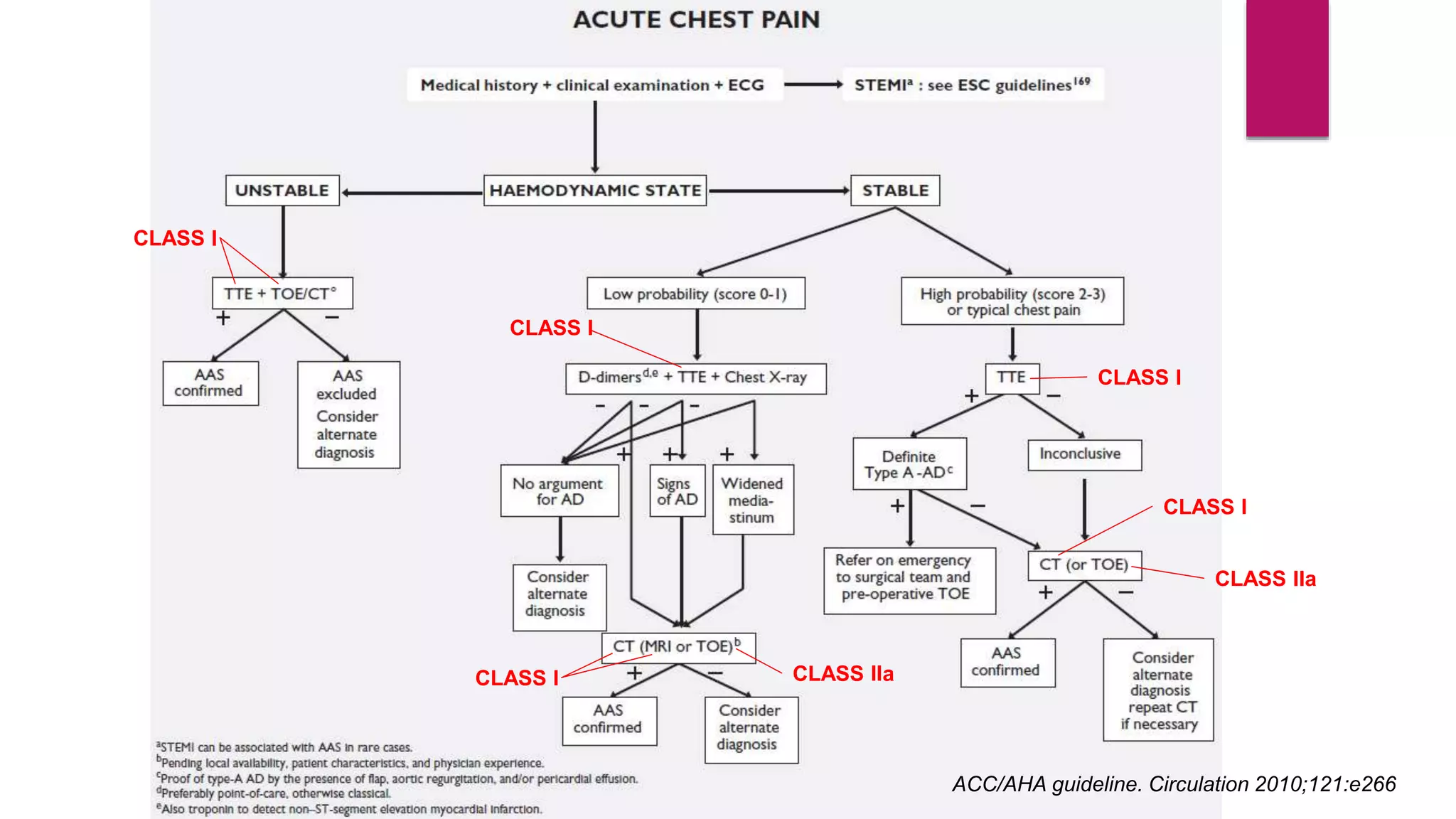
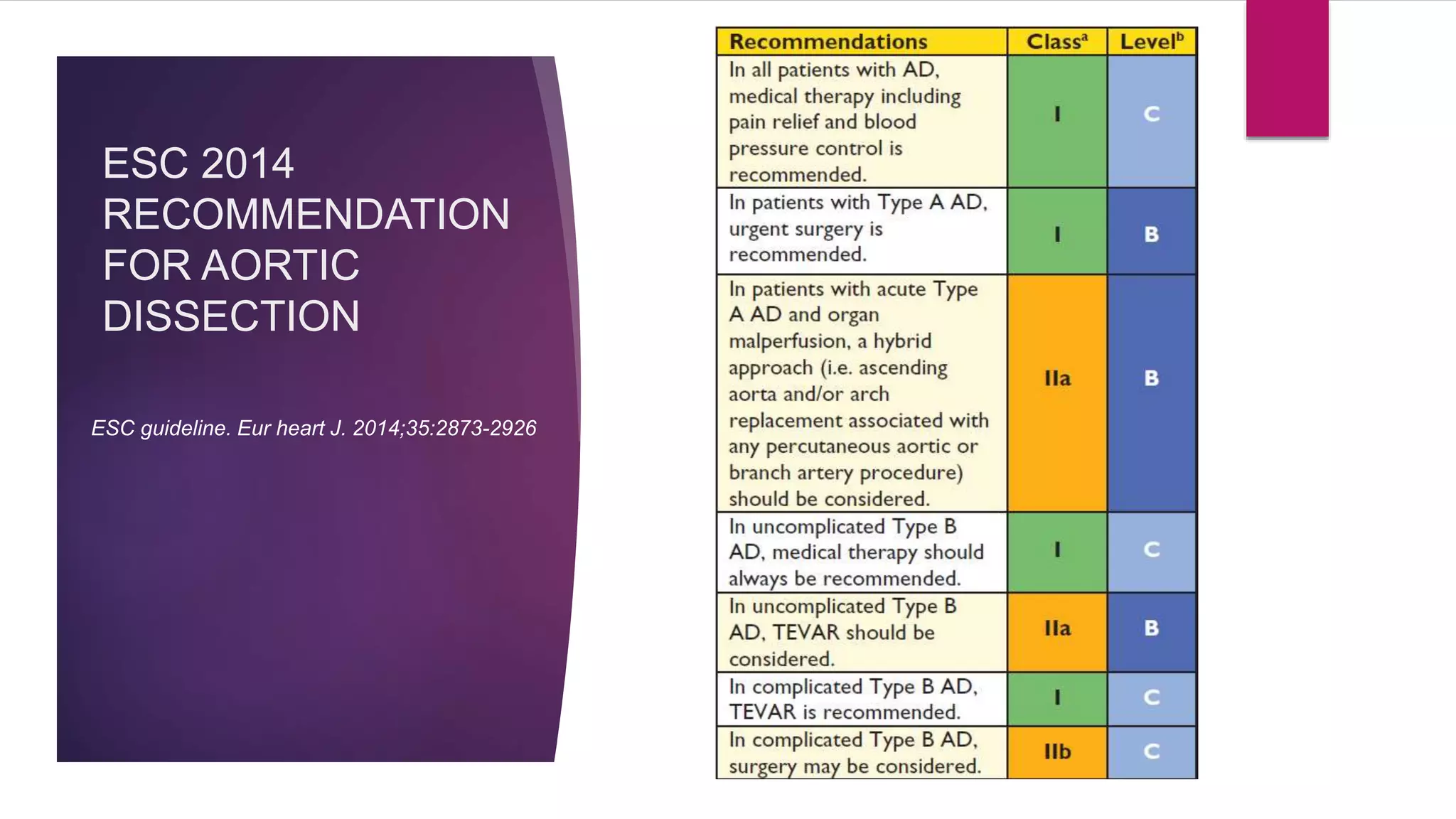
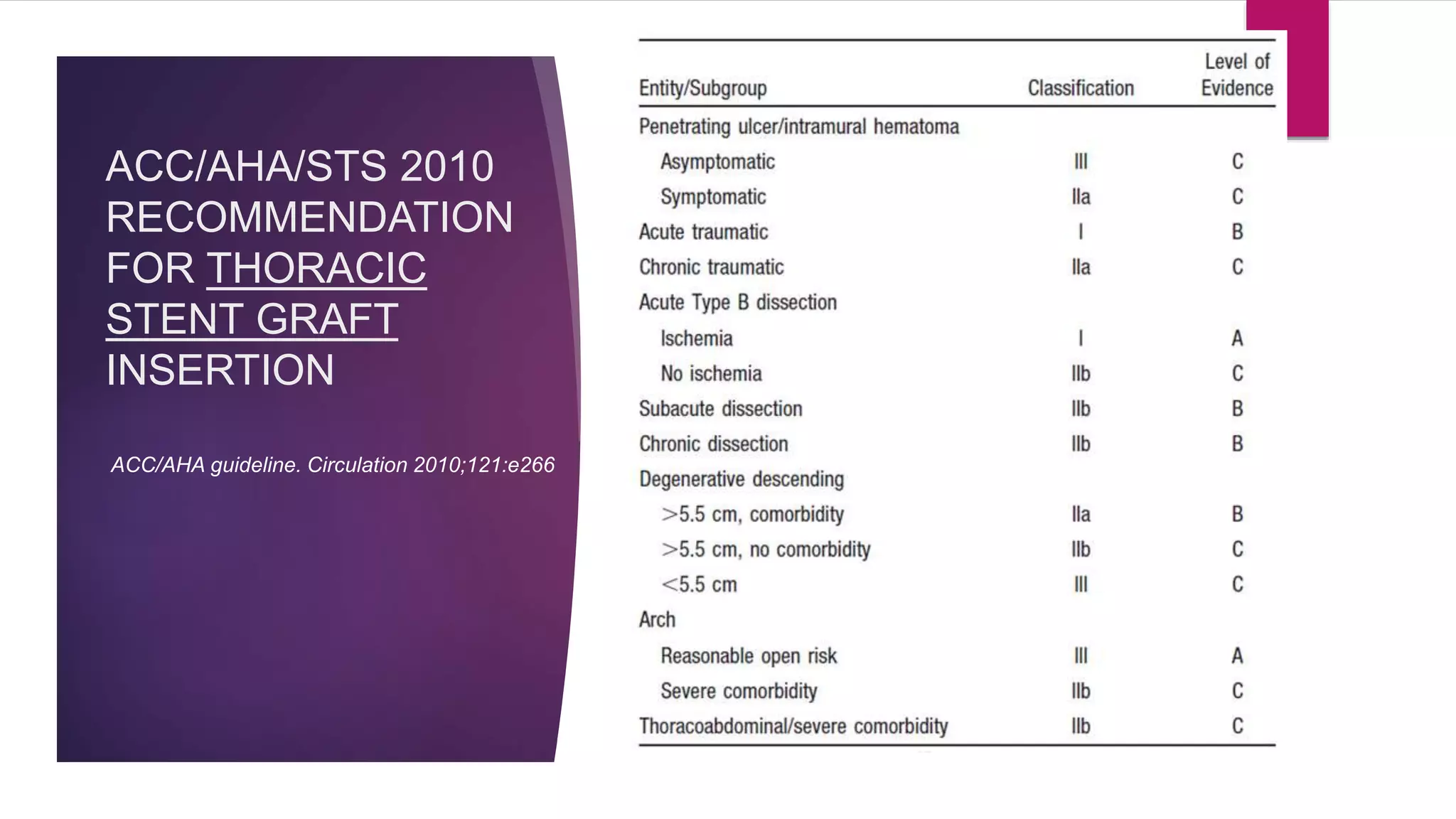
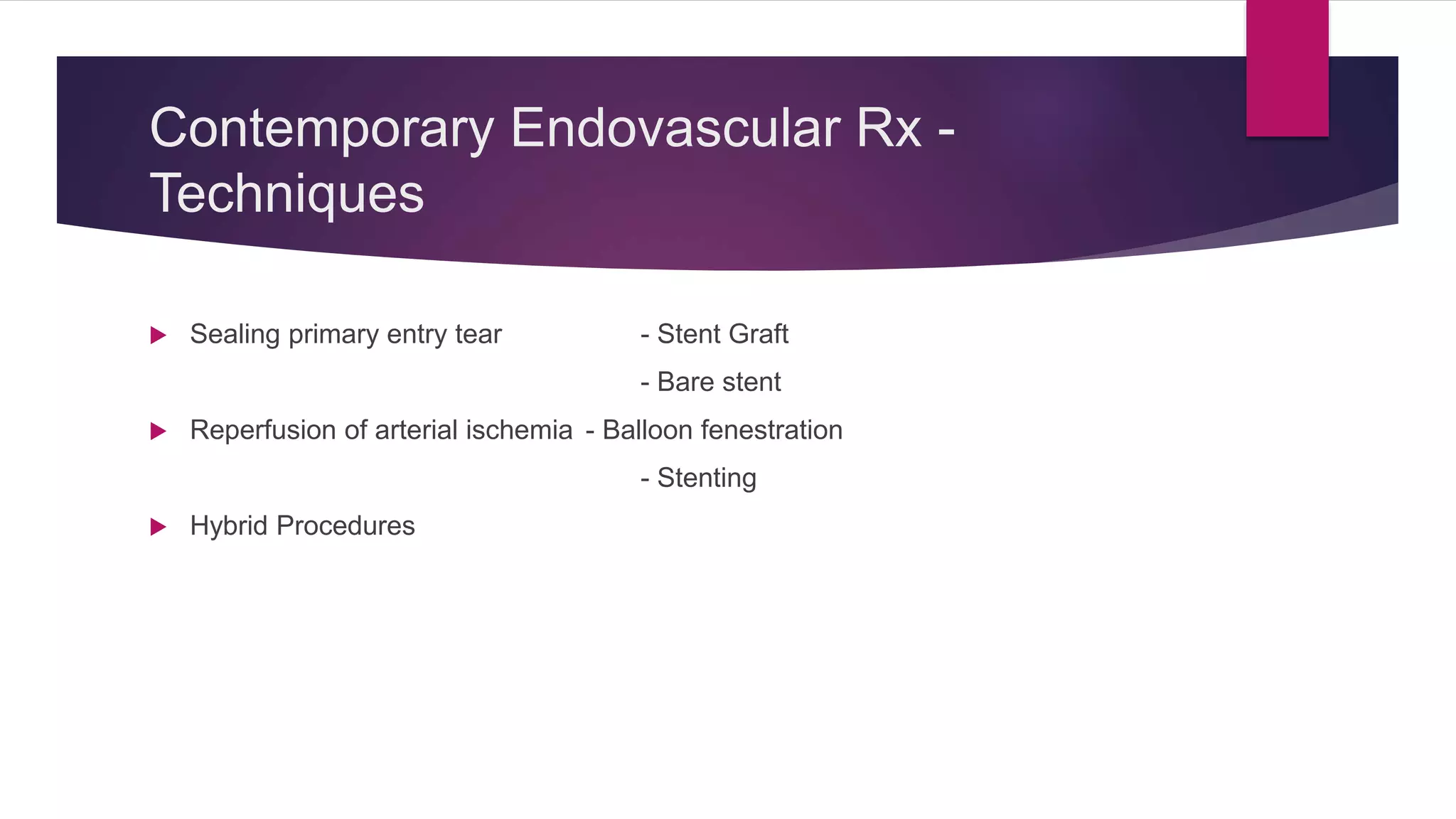
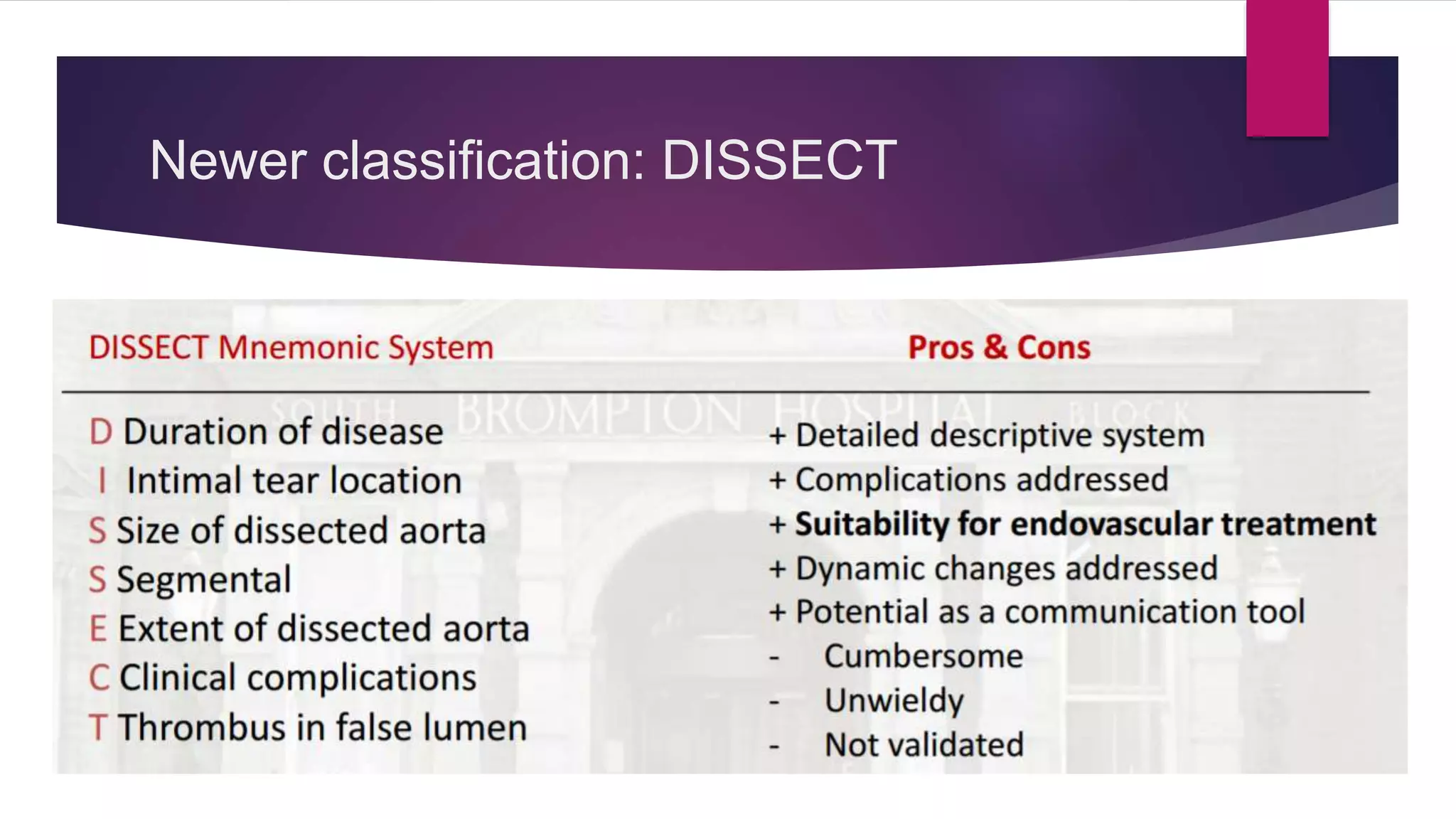
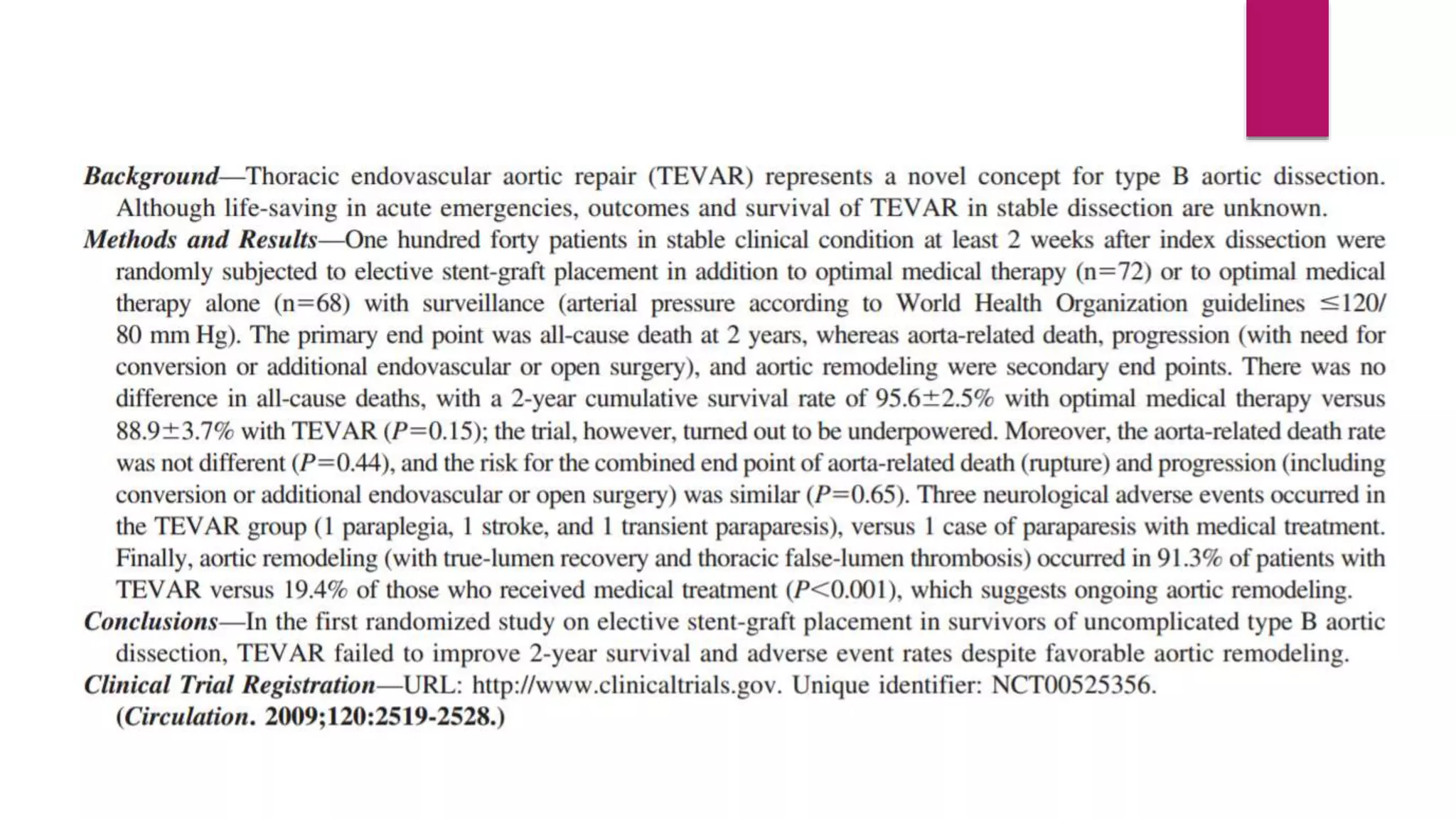
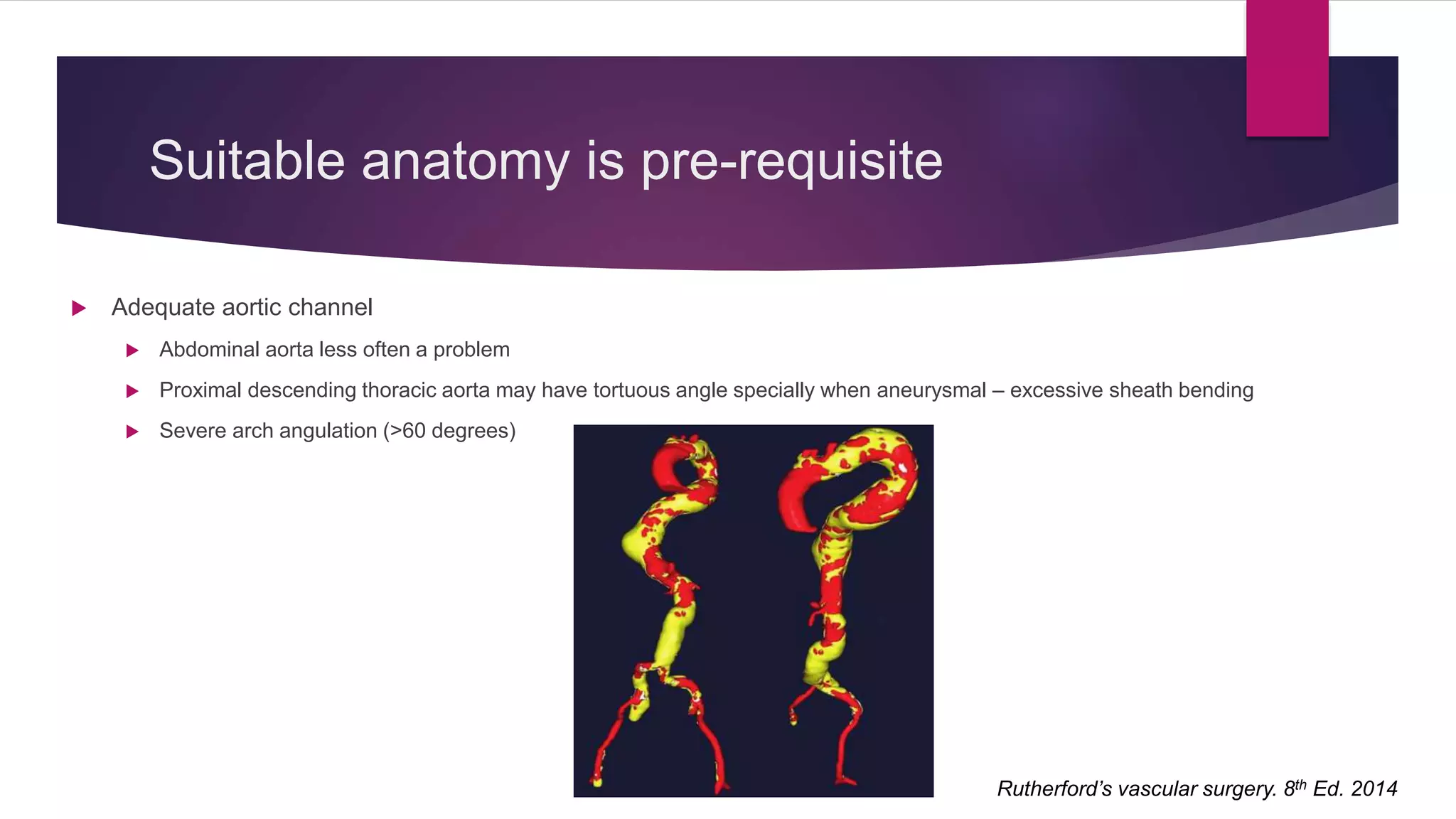
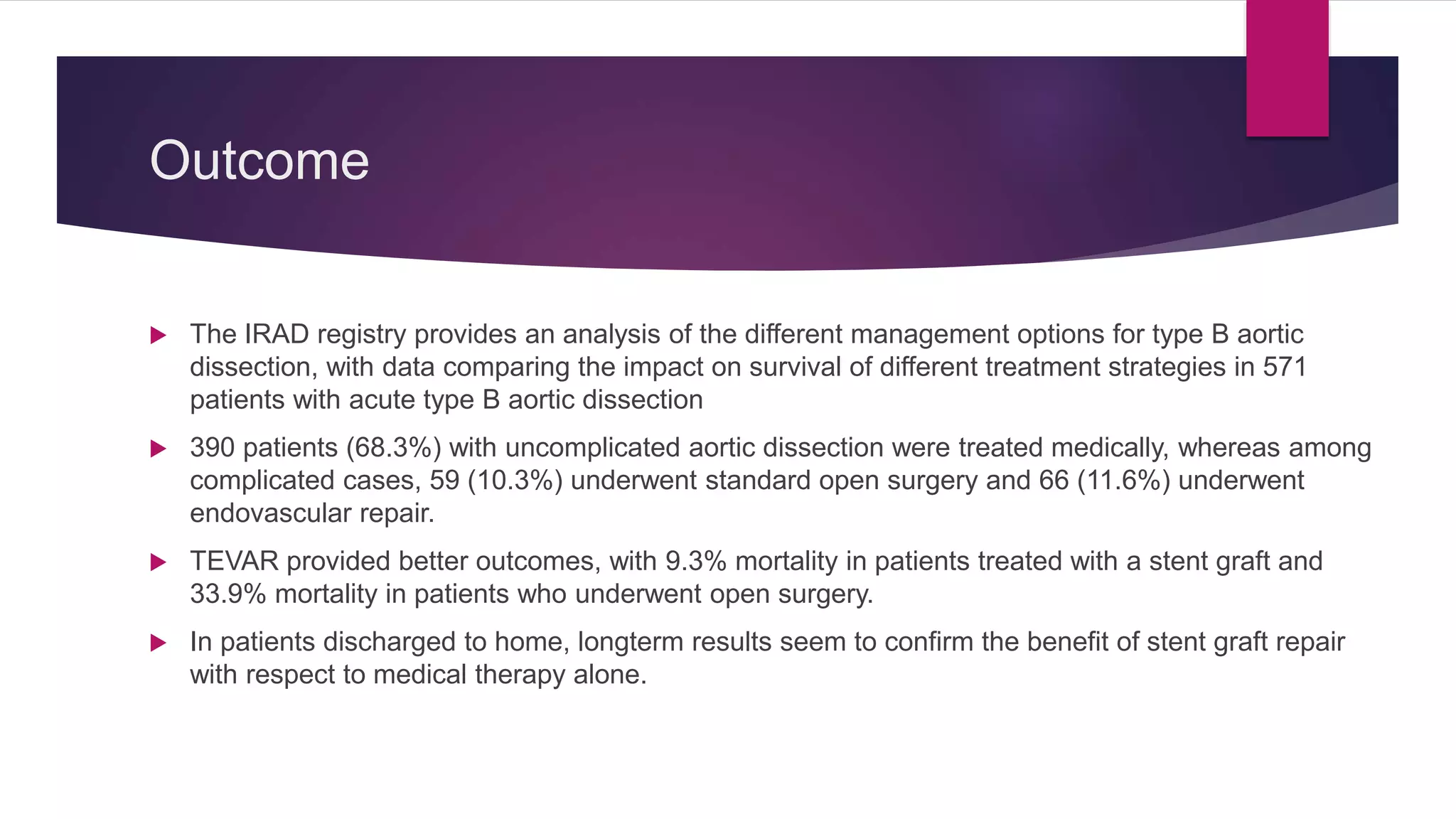
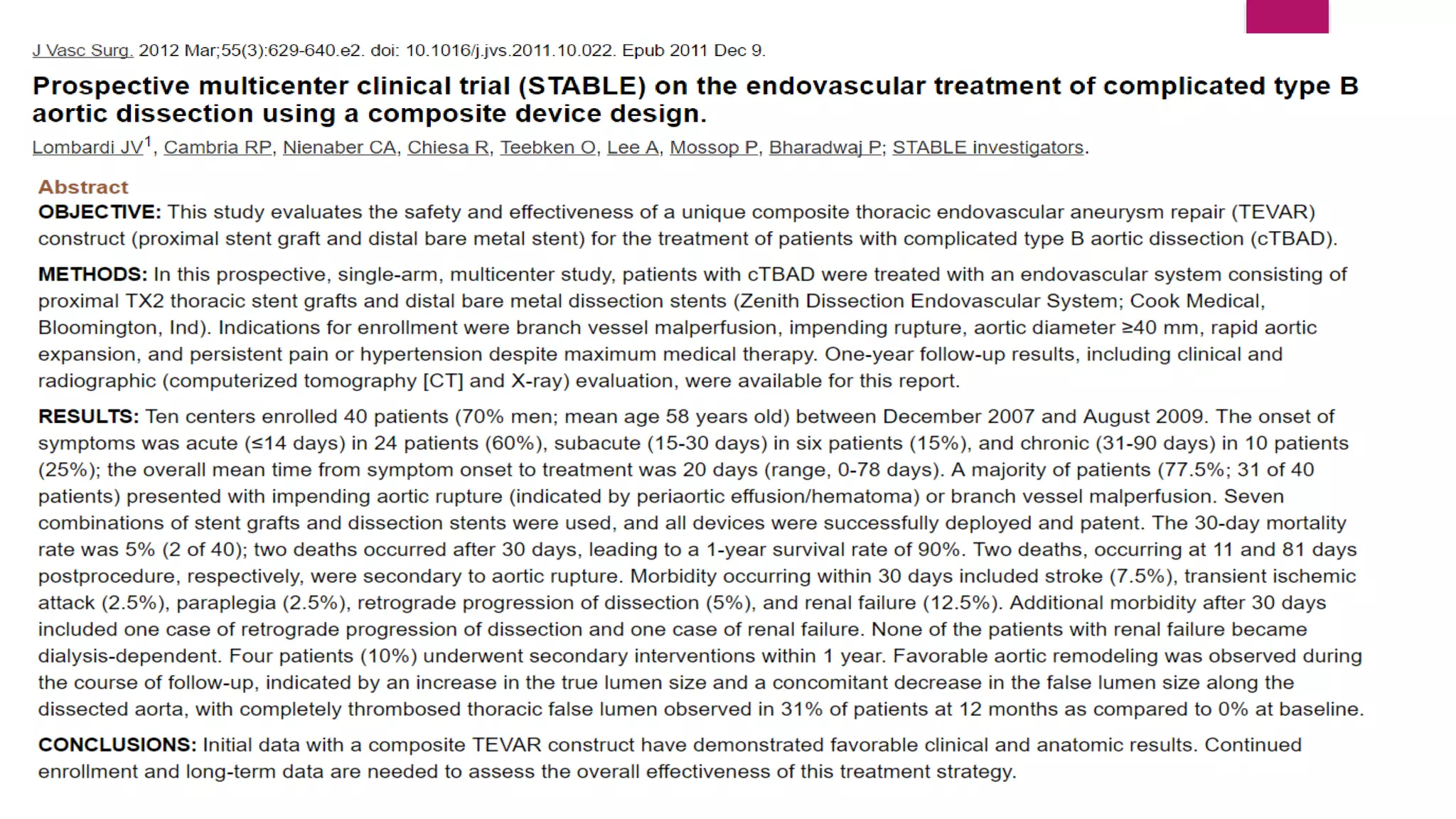
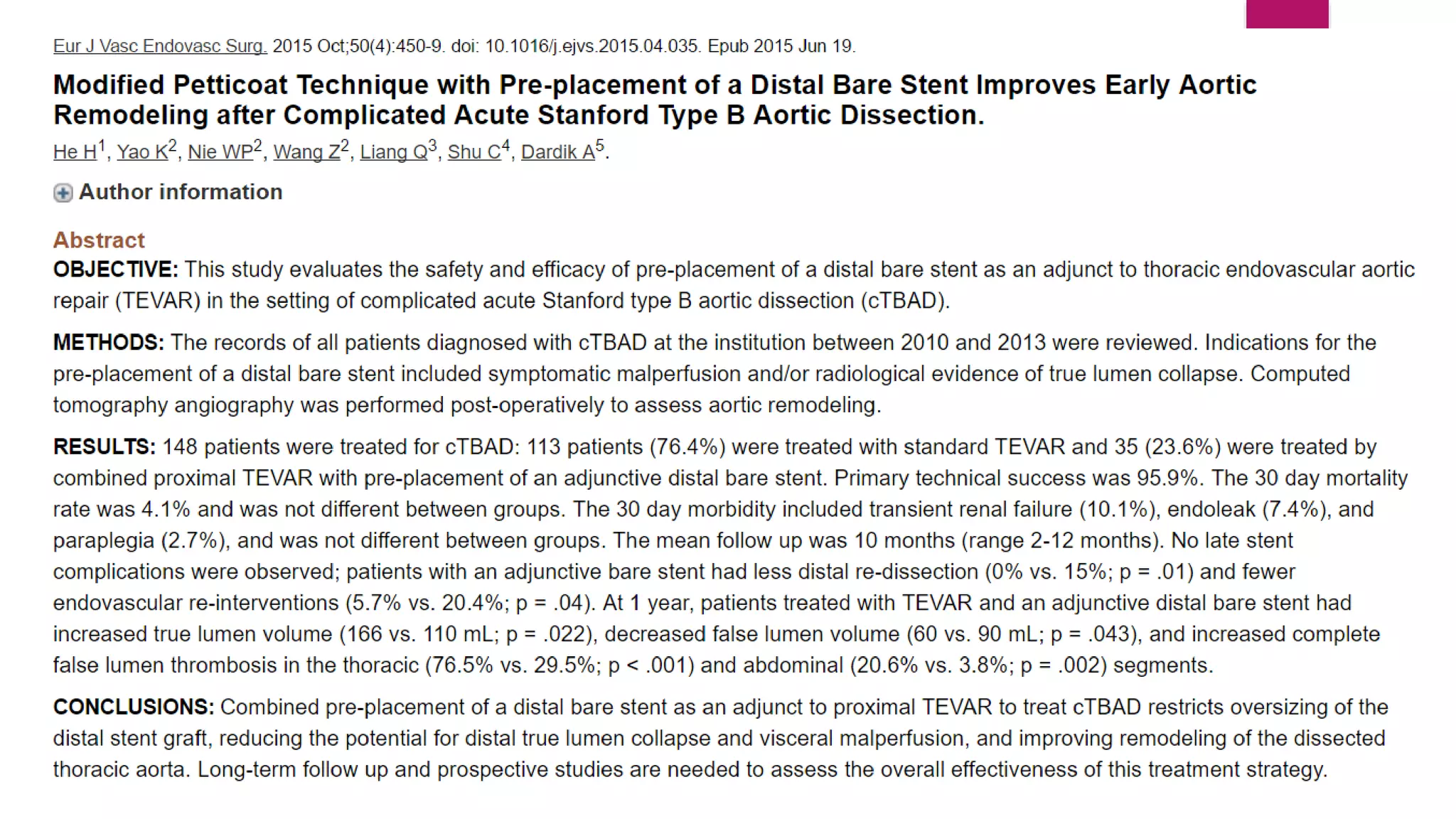
The document discusses endovascular treatment of aortic dissection. It begins with an introduction to aortic dissection, including definitions, classifications, epidemiology, clinical presentation, and natural history. It then discusses the diagnosis and imaging of aortic dissection. Medical and surgical management strategies are reviewed. Endovascular techniques for treating various types of aortic dissection are summarized. Key considerations for endovascular stent grafting as an alternative to open surgery are outlined.