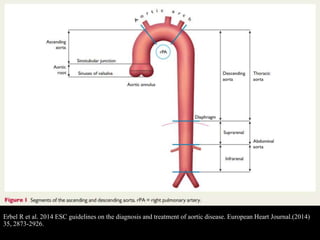
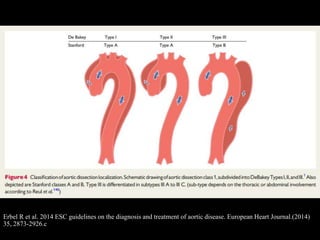
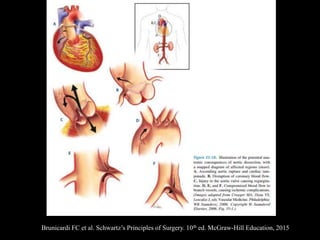
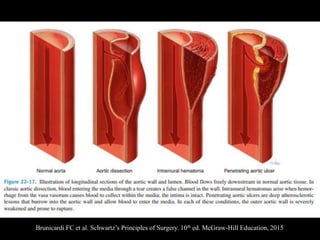
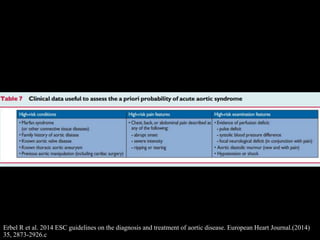
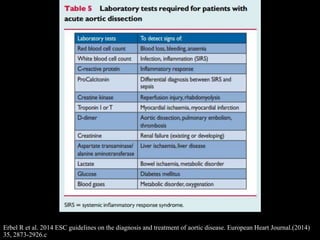
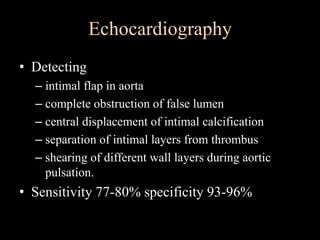
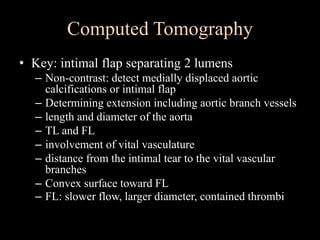
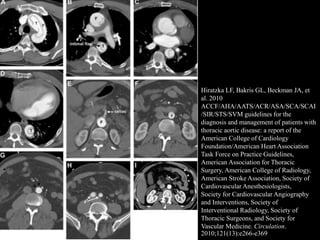
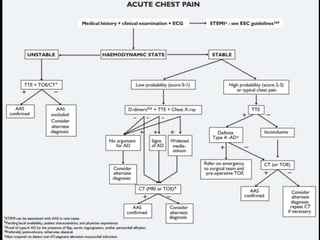
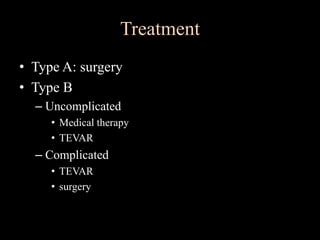
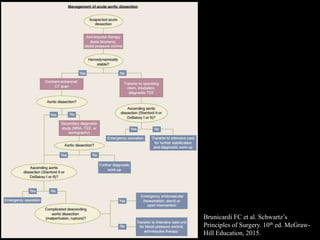
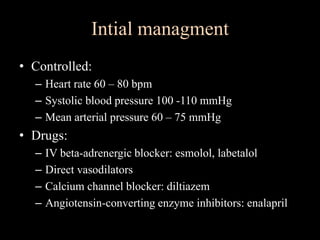
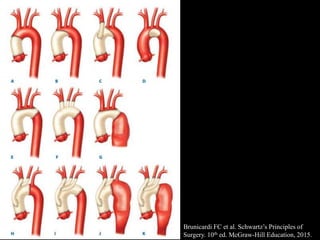
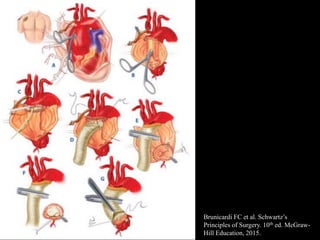
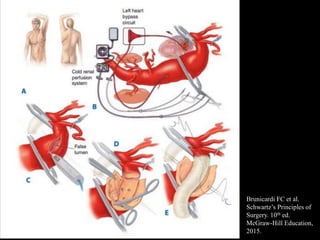
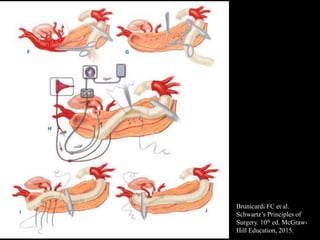
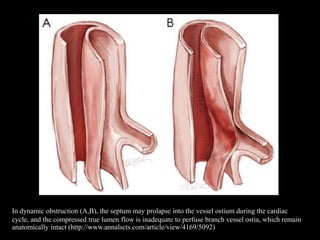
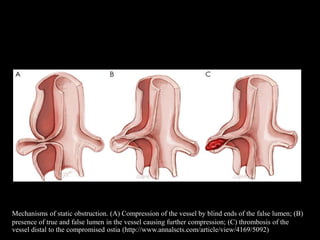
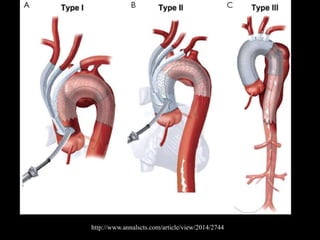
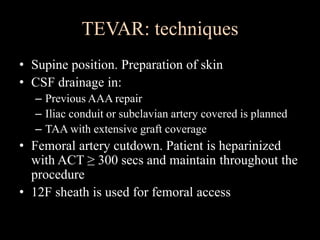
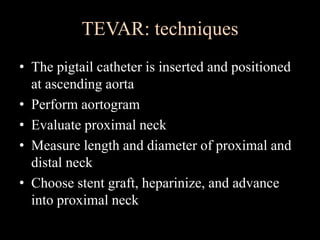
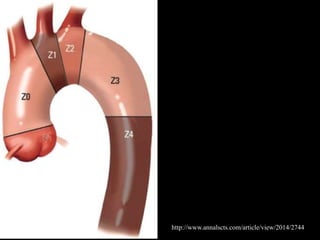
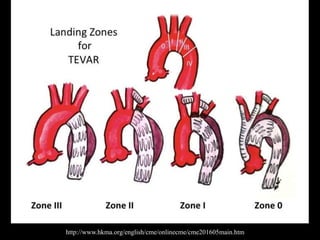
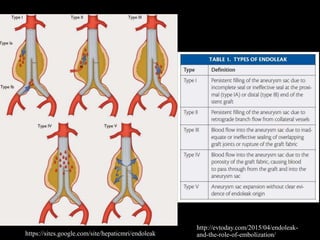
Aortic dissection is a life-threatening condition where the inner layer of the aorta tears, allowing blood to flow between the layers. It is classified as type A if the ascending aorta is involved and type B if it is isolated to the descending aorta. Type A requires emergency surgery while type B can often be treated medically or with TEVAR. Complications include malperfusion, rupture, and aortic expansion which may require intervention. Imaging plays a key role in diagnosis and management. Treatment aims to seal the entry tear, relieve malperfusion, and prevent further complications through control of blood pressure and heart rate.