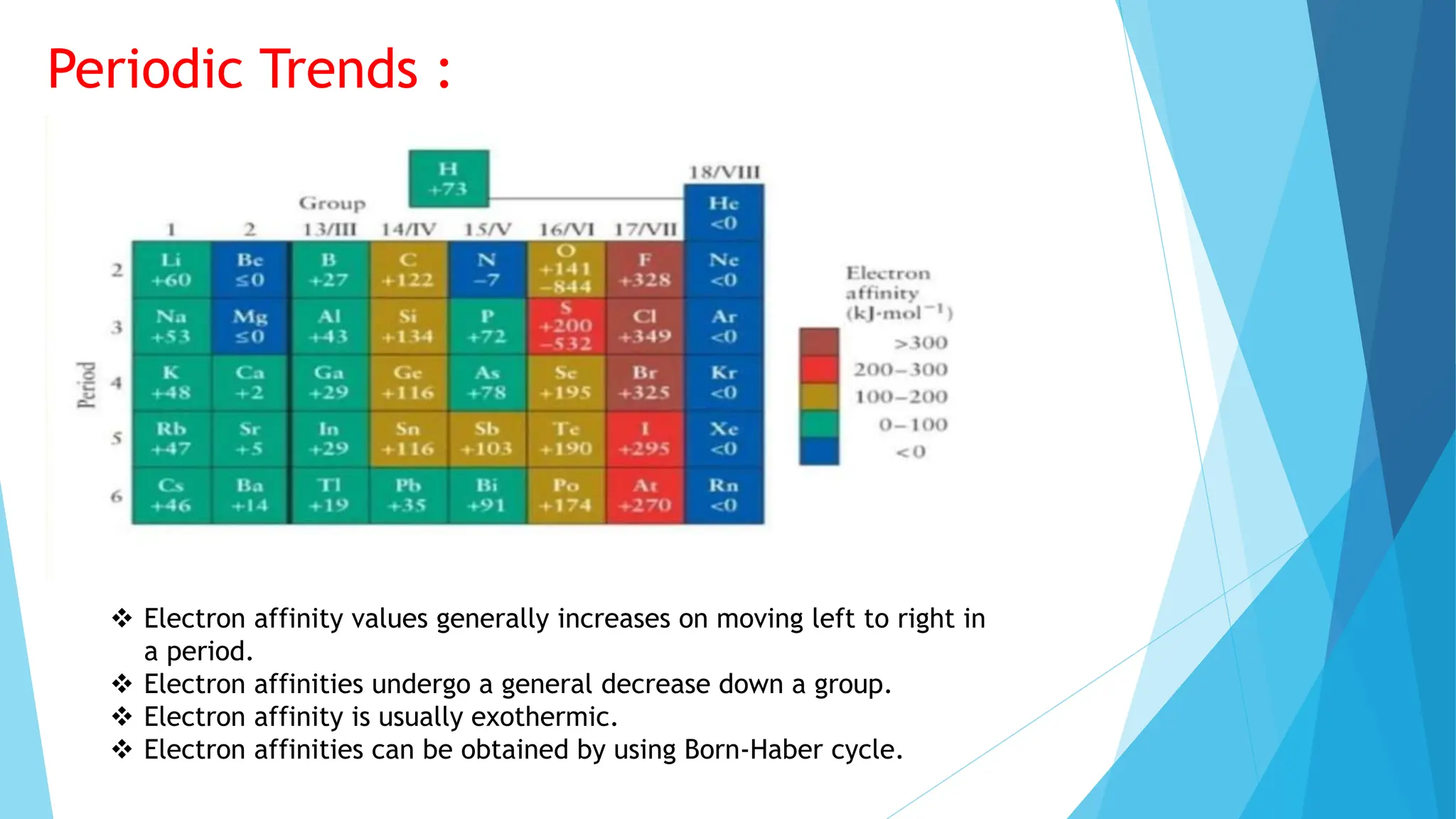
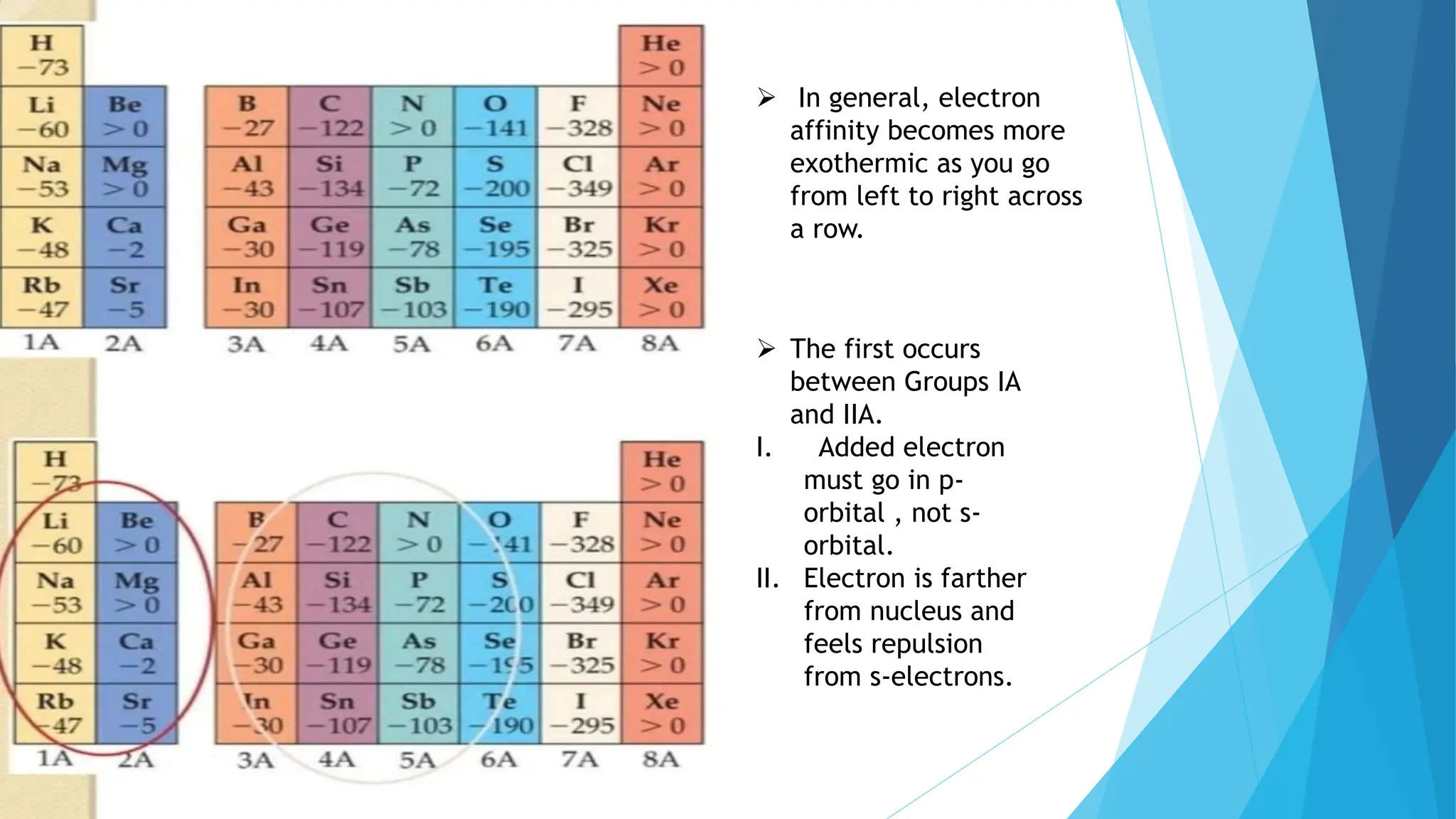
The document discusses electron affinity, defined as the energy change when an electron is added to a neutral atom to form a negative ion. It outlines factors affecting electron affinity including nuclear charge, atomic size, and electron configuration, noting that electron affinity generally increases across a period and decreases down a group. Additionally, it describes how electron affinity is often an exothermic process and can be analyzed using the Born-Haber cycle.