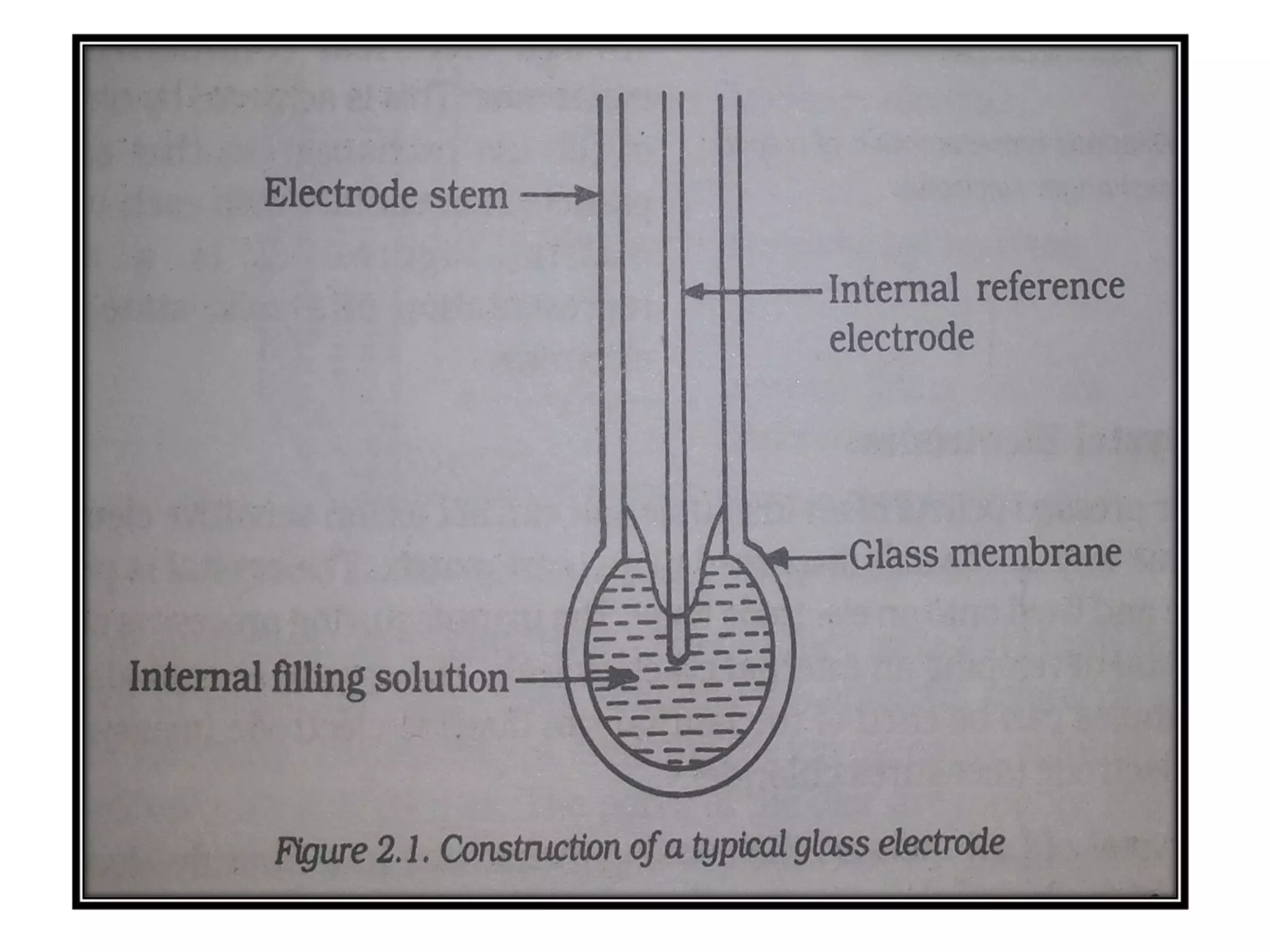
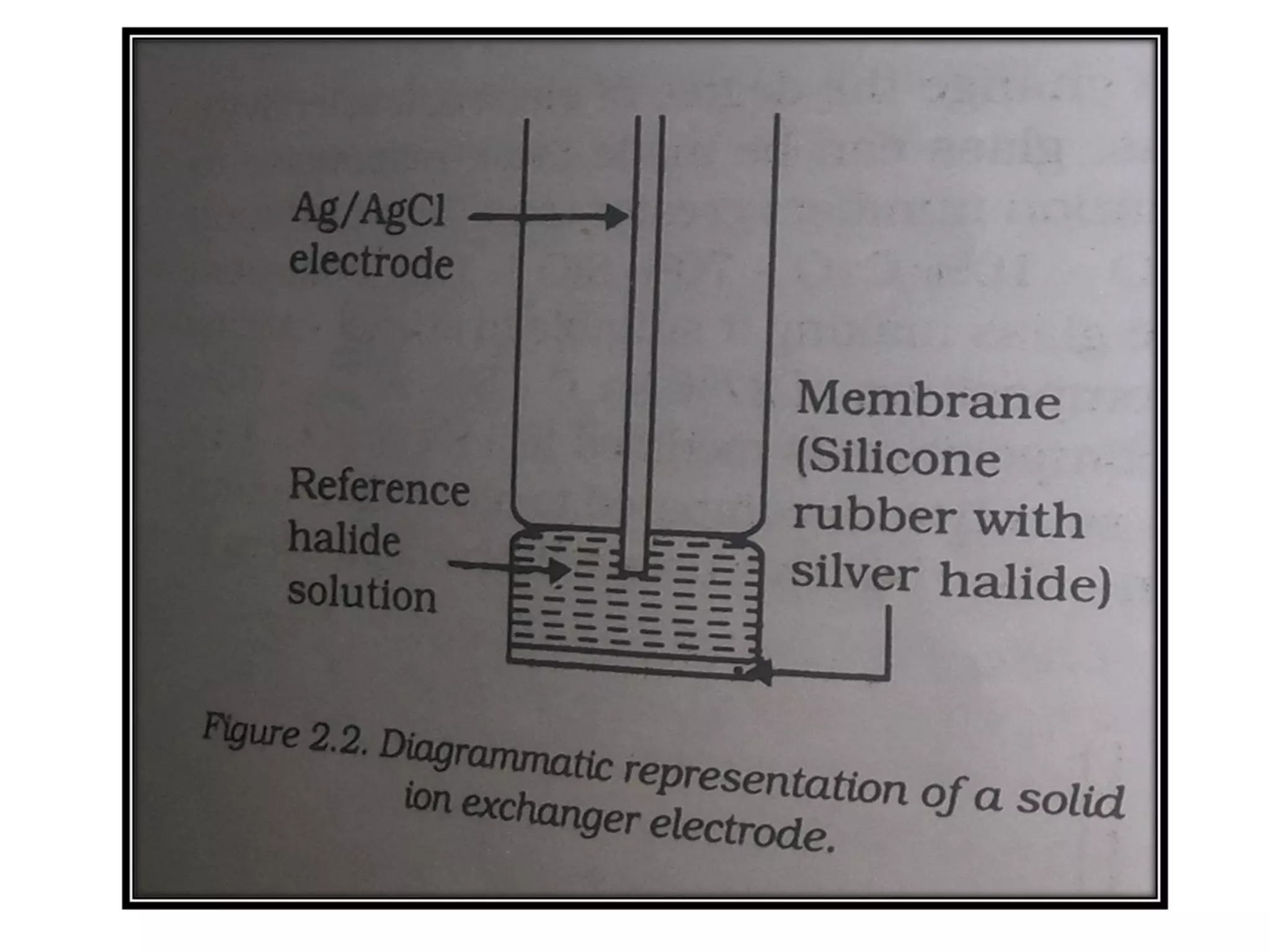
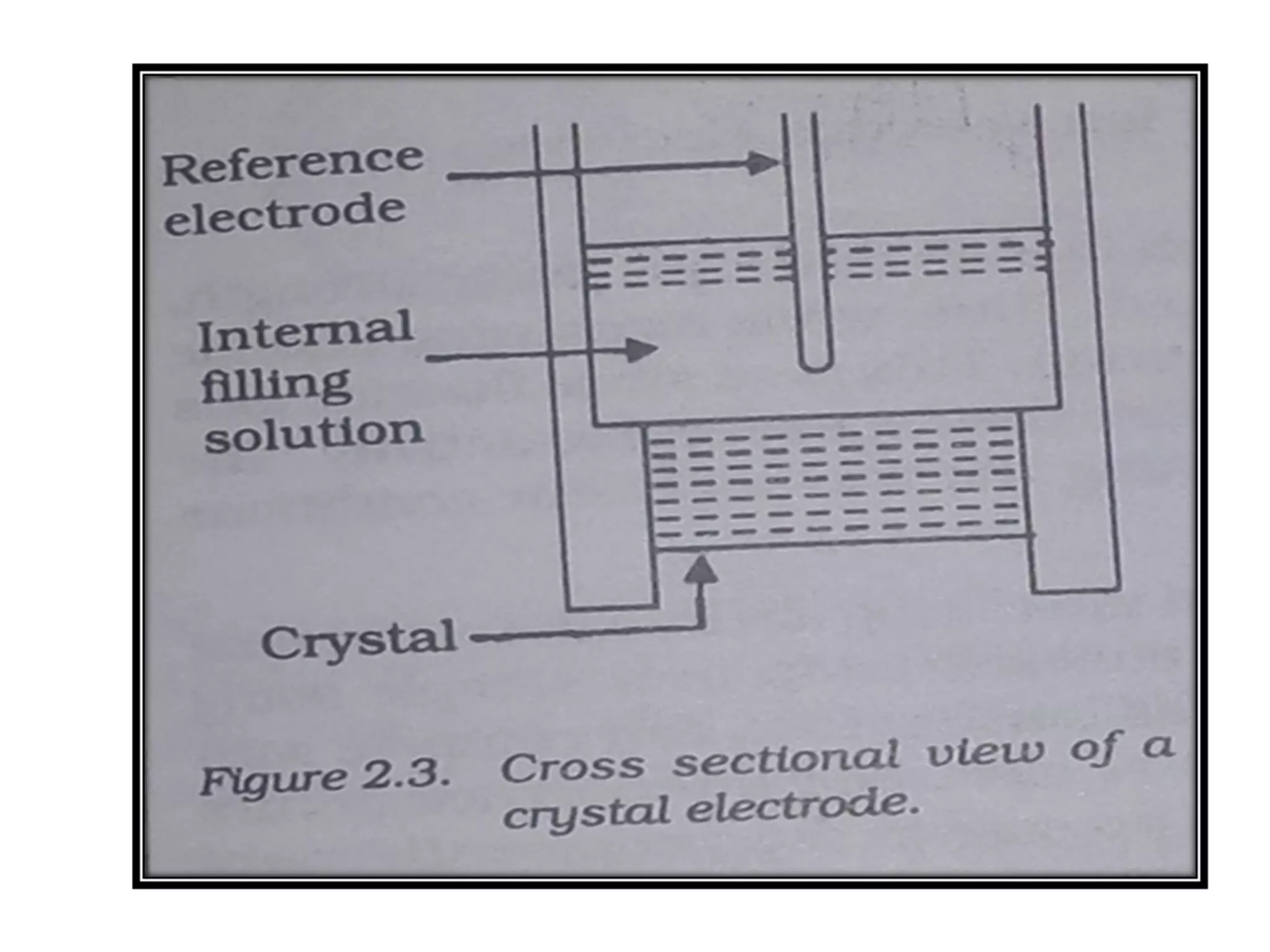
Ion selective electrodes (ISEs) are important tools in measuring the activity of metal ions, such as Na+, K+, and Ca2+, crucial for various cellular processes. They operate on principles similar to pH electrodes, incorporating an ion-selective membrane to detect specific ions and come in various types, including glass and solid-state electrodes. ISEs have diverse applications, including in diagnostics and studying ion transport in biological systems, and they are favored for their wide response range, low cost, and insensitivity to color or turbidity in samples.