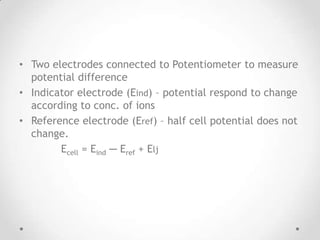
This document discusses potentiometry and ion selective electrodes. It begins by explaining that potentiometry measures the potential of an electrochemical cell under static conditions without drawing current. An ion selective electrode uses a selective membrane to measure the concentration of specific ions based on the potential difference between an indicator and reference electrode. The document then describes different types of reference electrodes, indicator electrodes, and ion selective electrodes like glass membrane, solid state, liquid membrane and gas sensing electrodes. It concludes by discussing applications in clinical chemistry, environmental analysis and food processing and advantages like speed and low cost and limitations like precision and interference issues.