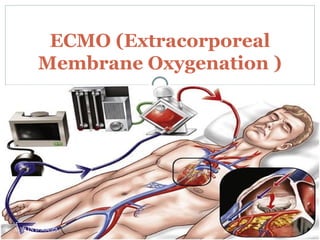
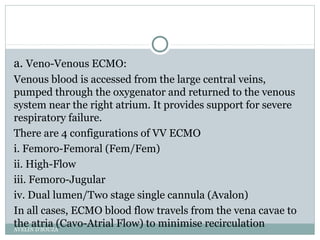
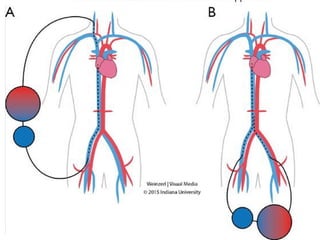
Extracorporeal Membrane Oxygenation (ECMO) is a life-support technique used for patients with severe pulmonary or cardiac failure, acting as a temporary measure while awaiting organ recovery. The document outlines the history, configurations (veno-venous and veno-arterial), indications for use, and potential complications associated with ECMO. It emphasizes the importance of patient monitoring, management of anticoagulation, and assessment of weaning protocols based on organ recovery and patient status.