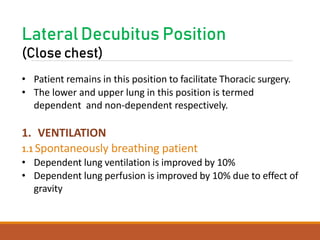
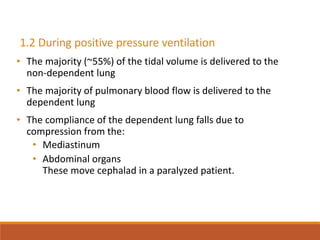
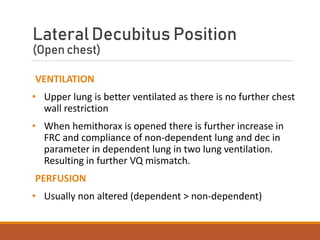
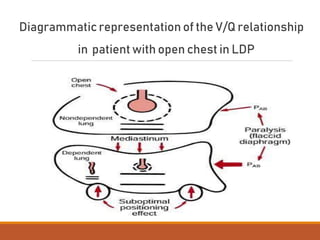
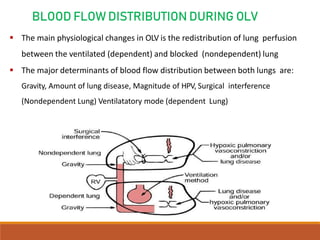
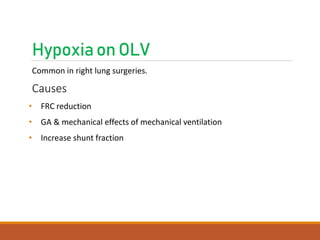
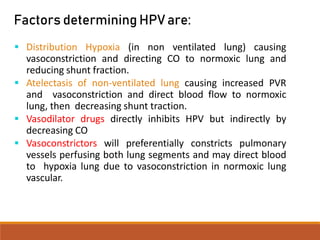
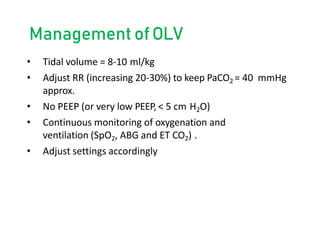
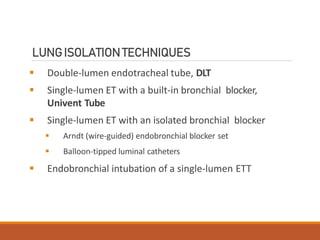
One Lung Ventilation (OLV) is a technique used during thoracic surgery to isolate one lung allowing it to function independently. There are several methods to achieve OLV including using a double lumen endotracheal tube, Univent tube, or endobronchial blockers. OLV provides benefits like protecting the healthy lung during surgery on the other lung but also causes physiological changes and risks like hypoxemia. Care must be taken to properly position the lung isolation device and monitor for complications during OLV.



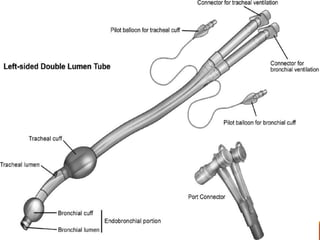
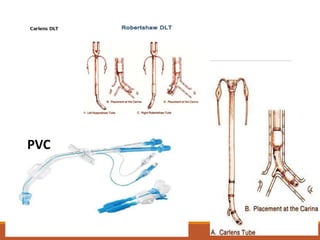
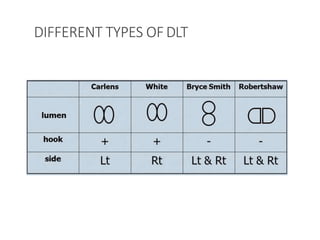
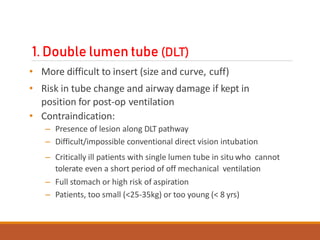



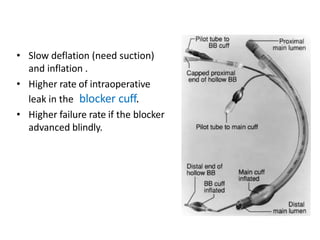
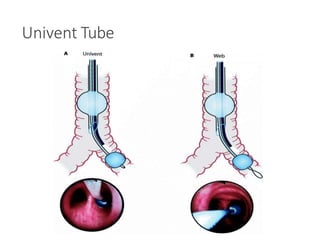
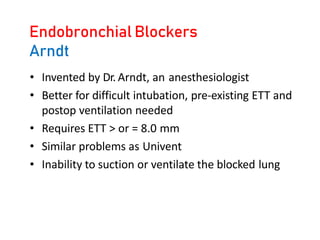
![Arndt endobronchial blocker
[WireguidedEndobronchialBlocker(WEB)]](https://image.slidesharecdn.com/7-230301133801-6e4f9869/85/7-One-lung-ventilation-pptx-22-320.jpg)