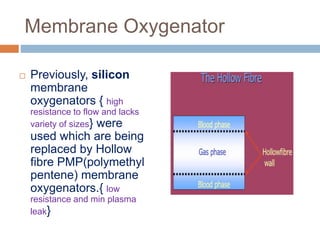
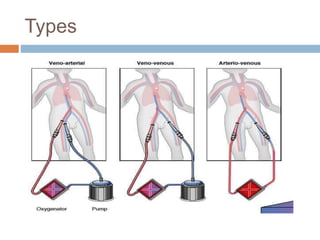
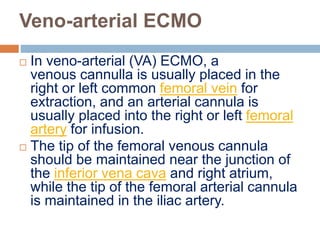
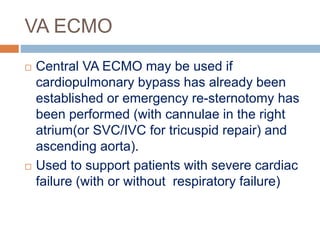
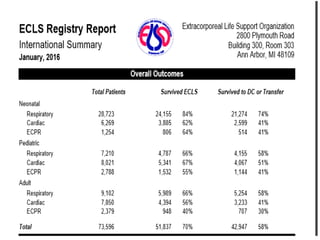
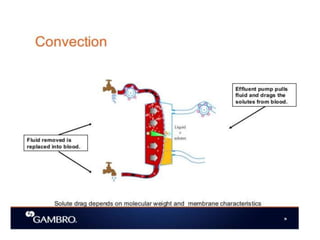
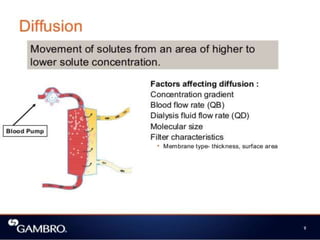
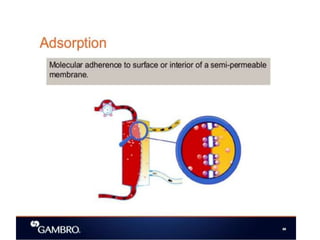
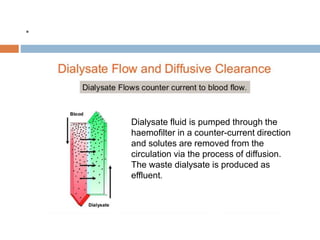
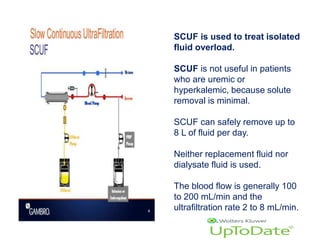
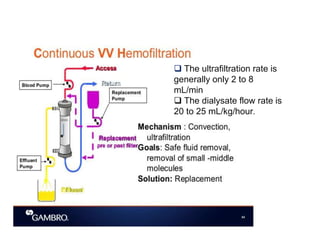
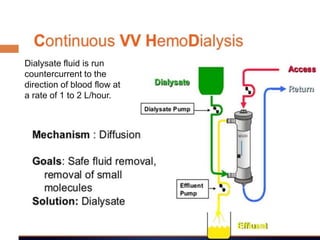
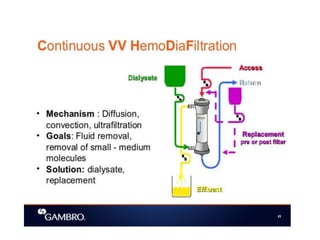
1. Extracorporeal membrane oxygenation (ECMO) and continuous renal replacement therapy (CRRT) are important life support therapies used in intensive care units.
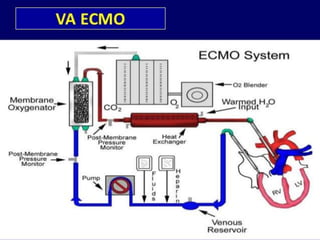
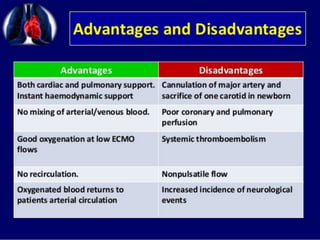
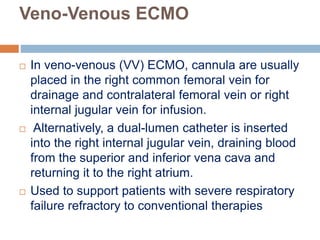
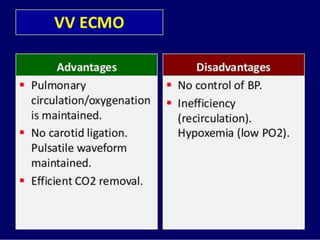
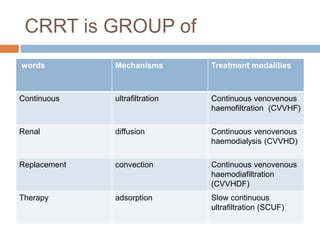
2. ECMO uses an external circuit to oxygenate blood and remove carbon dioxide, functioning as a bridge to recovery, transplant, or decision. CRRT slowly removes waste and fluid from the blood of patients with kidney failure or injury.
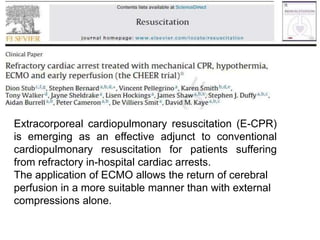
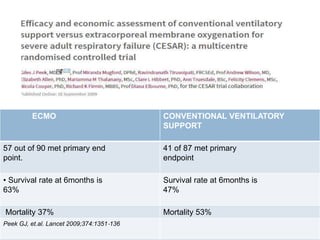
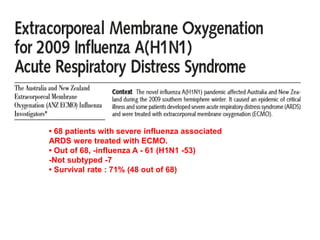
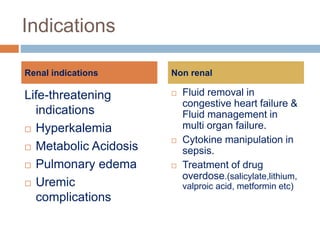
3. The document discusses the principles, indications, techniques, and complications of ECMO and CRRT, highlighting their roles in supporting critically ill patients with cardiac, respiratory, or renal issues.